Thiol/disulfide homeostasis in patients with ankylosing spondylitis
DOI:
https://doi.org/10.17305/bjbms.2016.1001Keywords:
Ankylosing spondylitis, disulfide, oxidative stress, thiolAbstract
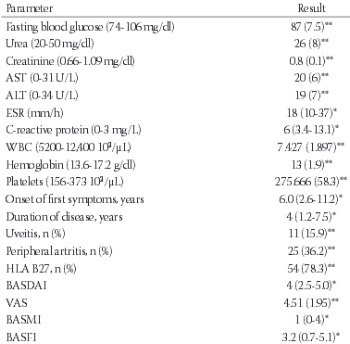
Ankylosing spondylitis (AS) is a chronic inflammatory disease. In many inflammatory diseases, increased production of pro-inflammatory cytokines is associated with an increase in oxidative stress mediators. Thiol/disulfide homeostasis is a marker for oxidative stress. The aim of this study was to examine the dynamic thiol/disulfide homeostasis in AS. Sixty-nine patients with AS and 60 age- and sex-matched controls were included in the study. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and visual analogue scale (VAS) were used to determine the disease activity. Native thiol, total thiol, and disulfide levels were measured with a novel automated method recently described by Erel and Neselioglu. The aforementioned method is also optionally manual spectrophotometric assay. The total thiol levels were significantly lower in the AS group compared with the control group (p = 0.03). When the patients were divided into active (n = 35) and inactive (n = 34) subgroups using BASDAI scores, the native plasma thiol and total thiol levels were significantly lower in the active AS patients compared to the inactive AS patients (p = 0.02, p = 0.03 respectively). There was a negative correlation between the plasma native thiol levels and VAS, BASDAI scores. Thiol/disulfide homeostasis may be used for elucidating the effects of oxidative stress in AS. Understanding the role of thiol/disulfide homeostasis in AS might provide new therapeutic intervention strategies for patients.
Downloads
References
Khan MA. Ankylosing spondylitis: Clinical features of ankylosing spondylitis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblah ME, Weisman MH, editors. Rheumatology, 3rd ed. Spain: Mosby; 2003. p. 1161-81.
Khan MA, Ball EJ. Genetic aspects of ankylosing spondylitis. Best Pract Res Clin Rheumatol 2002;16(4):675-90. http://dx.doi.org/10.1053/berh.2002.0243, http://dx.doi.org/10.1016/S1521-6942(02)90243-3.
Köse K, Yazici C, Cambay N, Ascioglu O, Dogan P. Lipid peroxidation and erythrocyte antioxidant enzymes in patients with Behçet's disease. Tohoku J Exp Med 2002;197(1):9-16. http://dx.doi.org/10.1620/tjem.197.9.
Karatas F, Ozates I, Canatan H, Halifeoglu I, Karatepe M, Colakt R. Antioxidant status and lipid peroxidation in patients with rheumatoid arthritis. Indian J Med Res 2003;118:178-81.
Ozgocmen S, Sogut S, Ardicoglu O, Fadillioglu E, Pekkutucu I, Akyol O. Serum nitric oxide, catalase, superoxide dismutase, and malondialdehyde status in patients with ankylosing spondylitis. Rheumatol Int 2004;24(2):80-3. http://dx.doi.org/10.1007/s00296-003-0335-y.
Yazici C, Köse K, Calis M, Kuzugüden S, Kirnap M. Protein oxidation status in patients with ankylosing spondylitis. Rheumatology (Oxford) 2004;43(10):1235-9. http://dx.doi.org/10.1093/rheumatology/keh317.
Yildirim K, Karatay S, Güreser G, Kızıltunç A, Uğur M, Şenel K. Antioxidantenzymes capacity in patients with rheumatoid arthritis: The relationship with disease activity score. Romatizma 2004;19:81-6.
Sarban S, Kocyigit A, Yazar M, Isikan UE. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin Biochem 2005;38(11):981-6. http://dx.doi.org/10.1016/j.clinbiochem.2005.08.003.
Karakoc M, Altindag O, Keles H, Soran N, Selek S. Serum oxidative-antioxidative status in patients with ankylosing spondylitis. Rheumatol Int 2007;27(12):1131-4. http://dx.doi.org/10.1007/s00296-007-0352-3.
LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum 2001;44(9):2078-83. http://dx.doi.org/10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J.
Horton JW. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology 2003;189(1-2):75-88. http://dx.doi.org/10.1016/S0300-483X(03)00154-9.
Biasi D, Carletto A, Caramaschi P, Bellavite P, Andrioli G, Caraffi M, et al. Neutrophil functions, spondylarthropathies and HLA-B27: A study of 43 patients. Clin Exp Rheumatol 1995;13(5):623-7.
Stichtenoth DO, Wollenhaupt J, Andersone D, Zeidler H, Frölich JC. Elevated serum nitrate concentrations in active spondyloarthropathies. Br J Rheumatol 1995;34(7):616-9. http://dx.doi.org/10.1093/rheumatology/34.7.616.
Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem 1989;58:79-110. http://dx.doi.org/10.1146/annurev.bi.58.070189.000455.
Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol 2001;54(3):176-86. http://dx.doi.org/10.1136/jcp.54.3.176.
Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochem Pharmacol 2006;71(5):551-64. http://dx.doi.org/10.1016/j.bcp.2005.10.044.
Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 2010;48(6):749-62. http://dx.doi.org/10.1016/j.freeradbiomed.2009.12.022.
Matteucci E, Giampietro O. Thiol signalling network with an eye to diabetes. Molecules 2010;15(12):8890-903. http://dx.doi.org/10.3390/molecules15128890.
Tetik S, Ahmad S, Alturfan AA, Fresko I, Disbudak M, Sahin Y, et al. Determination of oxidant stress in plasma of rheumatoid arthritis and primary osteoarthritis patients. Indian J Biochem Biophys 2010;47(6):353-8.
Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med 2011;50(4):495-509. http://dx.doi.org/10.1016/j.freeradbiomed.2010.11.029.
Kuo LM, Kuo CY, Lin CY, Hung MF, Shen JJ, Hwang TL. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules 2014;19(3):3327-44. http://dx.doi.org/10.3390/molecules19033327.
Prabhu A, Sarcar B, Kahali S, Yuan Z, Johnson JJ, Adam KP, et al. Cysteine catabolism: A novel metabolic pathway contributing to glioblastoma growth. Cancer Res 2014;74(3):787-96. http://dx.doi.org/10.1158/0008-5472.CAN-13-1423.
Mourad T, Min KL, Steghens JP. Measurement of oxidized glutathione by enzymatic recycling coupled to bioluminescent detection. Anal Biochem 2000;283(2):146-52. http://dx.doi.org/10.1006/abio.2000.4659.
Carru C, Deiana L, Sotgia S, Pes GM, Zinellu A. Plasma thiols redox status by laser-induced fluorescence capillary electrophoresis. Electrophoresis 2004;25(6):882-9. http://dx.doi.org/10.1002/elps.200305768.
Chen W, Zhao Y, Seefeldt T, Guan X. Determination of thiols and disulfides via HPLC quantification of 5-thio-2-nitrobenzoic acid. J Pharm Biomed Anal 2008;48(5):1375-80. http://dx.doi.org/10.1016/j.jpba.2008.08.033.
Glowacki R, Bald E. Fully automated method for simultaneous determination of total cysteine, cysteinylglycine, glutathione and homocysteine in plasma by HPLC with UV absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877(28):3400-4. http://dx.doi.org/10.1016/j.jchromb.2009.06.012.
Winther JR, Thorpe C. Quantification of thiols and disulfides. Biochim Biophys Acta 2014;1840(2):838-46. http://dx.doi.org/10.1016/j.bbagen.2013.03.031.
Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem 2014;47(18):326-32. http://dx.doi.org/10.1016/j.clinbiochem.2014.09.026.
Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27(4):361-8. http://dx.doi.org/10.1002/art.1780270401.
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21(12):2286-91.
Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: The development of the bath ankylosing spondylitis functional ındex. J Rheumatol 1994;21(12):2281-5.
Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17(1):45-56. http://dx.doi.org/10.1016/0304-3959(83)90126-4.
Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS metrology index. J Rheumatol 1994;21(9):1694-8.
Il'in MV, Mal'tseva PA, Rosanov DV, Volkova AS, Khrustalev AO. Changes in oxidative stress and apoptosis parameters of neutrophils in rheumatoid diseases. Zh Mikrobiol Epidemiol Immunobiol 2012;(1):89-92.
Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int 2000;58(6):2571-8. http://dx.doi.org/10.1046/j.1523-1755.2000.00443.x.
Kundi H, Erel Ö, Balun A, Çiçekçioglu H, Cetin M, Kiziltunç E, et al. Association of thiol/disulfide ratio with syntax score in patients with NSTEMI. Scand Cardiovasc J 2015;49(2):95-100. http://dx.doi.org/10.3109/14017431.2015.1013153.
Eren Y, Dirik E, Neselioglu S, Erel Ö. Oxidative stress and decreased thiol level in patients with migraine: Cross-sectional study. Acta Neurol Belg 2015;115(4):643-9. http://dx.doi.org/10.1007/s13760-015-0427-y.
Erkus ME, Altiparmak IH, Akyuz AR, Demirbag R, Sezen Y, Gunebakmaz O, et al. The association between plasma thiol levels and left ventricular diastolic dysfunction in patient with hypertension. Scand J Clin Lab Invest. 2015;75(8):667-73. http://dx.doi.org/10.1016/j.amjcard.2015.01.076.
Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med 2009;47(10):1329-38. http://dx.doi.org/10.1016/j.freeradbiomed.2009.08.021.
Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem 2013;288(37):26489-96. http://dx.doi.org/10.1074/jbc.R113.462929.
Ates I, Kaplan M, Inan B, Alisik M, Erel O, Yilmaz N, et al. How does thiol/disulfide homeostasis change in prediabetic patients? Diabetes Res Clin Pract 2015;110(2):166-71. http://dx.doi.org/10.1016/j.diabres.2015.09.011.
Ates I, Ozkayar N, Inan B, Yilmaz FM, Topcuoglu C, Neselioglu S, et al. Dynamic thiol/disulphide homeostasis in patients with newly diagnosed primary hypertension. J Am Soc Hypertens 2016;10(2):159-66. http://dx.doi.org/10.1016/j.jash.2015.12.008.
Erkenekli K, Sanhal CY, Yucel A, Bicer CK, Erel O, Uygur D. Thiol/disulfide homeostasis in patients with idiopathic recurrent pregnancy loss assessed by a novel assay: Report of a preliminary study. J Obstet Gynaecol Res 2016;42(2):136-41. http://dx.doi.org/10.1111/jog.12860.