DL-2-amino-3-phosphonopropionic acid protects primary neurons from oxygen-glucose deprivation induced injury
DOI:
https://doi.org/10.17305/bjbms.2016.1553Keywords:
DL-2-amino-3-phosphonopropionic acid, cerebral infarction, oxygen-glucose deprivation, neuron viability, apoptosisAbstract
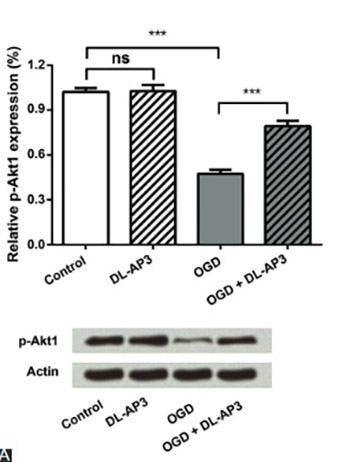
Cerebral infarction is a type of ischemic stroke and is one of the main causes of irreversible brain damage. Although multiple neuroprotective agents have been investigated recently, the potential of DL-2-amino-3-phosphonopropionic acid (DL-AP3) in treating oxygen-glucose deprivation (OGD)-induced neuronal injury, has not been clarified yet. This study was aimed to explore the role of DL-AP3 in primary neuronal cell cultures. Primary neurons were divided into four groups: (1) a control group that was not treated; (2) DL-AP3 group treated with 10 μM of DL-AP3; (3) OGD group, in which neurons were cultured under OGD conditions; and (4) OGD + DL-AP3 group, in which OGD model was first established and then the cells were treated with 10 μM of DL-AP3. Neuronal viability and apoptosis were measured using Cell Counting Kit-8 and flow cytometry. Expressions of phospho-Akt1 (p-Akt1) and cytochrome c were detected using Western blot. The results showed that DL-AP3 did not affect neuronal viability and apoptosis in DL-AP3 group, nor it changed p-Akt1 and cytochrome c expression (p > 0.05). In OGD + DL-AP3 group, DL-AP3 significantly attenuated the inhibitory effects of OGD on neuronal viability (p < 0.001), and reduced OGD induced apoptosis (p < 0.01). Additionally, the down-regulation of p-Akt1 and up-regulation of cytochrome c, induced by OGD, were recovered to some extent after DL-AP3 treatment (p < 0.05 or p < 0.001). Overall, DL-AP3 could protect primary neurons from OGD-induced injury by affecting the viability and apoptosis of neurons, and by regulating the expressions of p-Akt1 and cytochrome c.
Downloads
References
Liu JJ, Pan SY. Protective effects of estrogen combined with sevoflurane in an experimental model of cerebral infarction and focal cerebral ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci 2016;20(9):1839-44.
Hankey GJ. Potential new risk factors for ischemic stroke: what is their potential? Stroke 2006;37(8):2181-8. http://dx.doi.org/10.1161/01.STR.0000229883.72010.e4.
Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng R, et al. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J Transl Med 2011;9:105.
http://dx.doi.org/10.1186/1479-5876-9-105.
Wang S, Zhou J, Kang W, Dong Z, Wang H. Tocilizumab inhibits neuronal cell apoptosis and activates STAT3 in cerebral infarction rat model. Bosn J Basic Med Sci 2016;16(2):145-50. http://dx.doi.org/10.17305/bjbms.2016.853.
Onteddu SR, Goddeau RP Jr, Minaeian A, Henninger N. Clinical impact of leukoaraiosis burden and chronological age on neurological deficit recovery and 90-day outcome after minor ischemic stroke. J Neurol Sci 2015;359(1-2):418-23. http://dx.doi.org/10.1016/j.jns.2015.10.005.
Slomka M, Kuszczyk M, Lazarewicz JW, Makarewicz D. NMDA receptor antagonists MK-801 and memantine induce tolerance to oxygen and glucose deprivation in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp (Wars) 2014;74(4):396-404.
Kanthan R, Shuaib A, Griebel R, Miyashita H. Intracerebral human microdialysis. In vivo study of an acute focal ischemic model of the human brain. Stroke 1995;26(5):870-3. http://dx.doi.org/10.1161/01.STR.26.5.870.
Fisher M. Characterizing the target of acute stroke therapy. Stroke 1997;28(4):866-72. http://dx.doi.org/10.1161/01.STR.28.4.866.
Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993;262(5134):689-95. http://dx.doi.org/10.1126/science.7901908.
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999;283(5398):70-4. http://dx.doi.org/10.1126/science.283.5398.70.
Yun BR, Yang HJ, Weon JB, Lee J, Eom MR, Ma CJ. Neuroprotective properties of compounds extracted from Dianthus superbus L. against glutamate-induced cell death in HT22 cells. Pharmacogn Mag 2016;12(46):109-13.
http://dx.doi.org/10.4103/0973-1296.177905.
Kuszczyk M, Slomka M, Antkiewicz-Michaluk L, Salinska E, Lazarewicz JW. 1-Methyl-1,2,3,4-tetrahydroisoquinoline and established uncompetitive NMDA receptor antagonists induce tolerance to excitotoxicity. Pharmacol Rep 2010;62(6):1041-50. http://dx.doi.org/10.1016/S1734-1140(10)70366-2.
Ke T, Li R, Chen W. Inhibition of the NMDA receptor protects the rat sciatic nerve against ischemia/reperfusion injury. Exp Ther Med 2016;11(5):1563-72.
http://dx.doi.org/10.3892/etm.2016.3148.
Adachi N, Numakawa T, Kumamaru E, Itami C, Chiba S, Iijima Y, et al. Phencyclidine-induced decrease of synaptic connectivity via inhibition of BDNF secretion in cultured cortical neurons. Cereb Cortex 2013;23(4):847-58. http://dx.doi.org/10.1093/cercor/bhs074.
Xu SY, Wu YM, Ji Z, Gao XY, Pan SY. A modified technique for culturing primary fetal rat cortical neurons. J Biomed Biotechnol 2012;2012:803930. http://dx.doi.org/10.1155/2012/803930.
Gao XY, Huang JO, Hu YF, Gu Y, Zhu SZ, Huang KB, et al. Combination of mild hypothermia with neuroprotectants has greater neuroprotective effects during oxygen-glucose deprivation and reoxygenation-mediated neuronal injury. Sci Rep 2014;4:7091. http://dx.doi.org/10.1038/srep07091.
Wang Z, Yang P, Qi Y. Role of microRNA-134 in the neuroprotective effects of propofol against oxygen-glucose deprivation and related mechanisms. Int J Clin Exp Med 2015;8(11):20617-23.
Vieira M, Fernandes J, Carreto L, Anuncibay-Soto B, Santos M, Han J, et al. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol Dis 2014;68:26-36.
http://dx.doi.org/10.1016/j.nbd.2014.04.002.
Sheng R, Liu XQ, Zhang LS, Gao B, Han R, Wu YQ, et al. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy 2012;8(3):310-25. http://dx.doi.org/10.4161/auto.18673.
Pan XW, Zhao XH. In vitro proliferation and anti-apoptosis of the papain-generated casein and soy protein hydrolysates towards osteoblastic cells (hFOB1.19). Int J Mol Sci 2015;16(6):13908-20. http://dx.doi.org/10.3390/ijms160613908.
Zhang N, Su Y, Xu L. Targeting PKCepsilon by miR-143 regulates cell apoptosis in lung cancer. FEBS Lett 2013;587(22):3661-7. http://dx.doi.org/10.1016/j.febslet.2013.09.018.
Nam K, Oh S, Lee KM, Yoo SA, Shin I. CD44 regulates cell proliferation, migration, and invasion via modulation of c-Src transcription in human breast cancer cells. Cell Signal 2015;27(9):1882-94. http://dx.doi.org/10.1016/j.cellsig.2015.05.002.
Trape AP, Liu S, Cortes AC, Ueno NT, Gonzalez-Angulo AM. Effects of CDK4/6 inhibition in hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer cells with acquired resistance to paclitaxel. J Cancer 2016;7(8):947-56. http://dx.doi.org/10.7150/jca.14441.
Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology 2008;55(3):310-8. http://dx.doi.org/10.1016/j.neuropharm.2008.01.005.
Aboutaleb N, Shamsaei N, Rajabi H, Khaksari M, Erfani S, Nikbakht F, et al. Protection of hippocampal CA1 neurons against ischemia/reperfusion injury by exercise preconditioning via modulation of Bax/Bcl-2 ratio and prevention of caspase-3 activation. Basic Clin Neurosci 2016;7(1):21-9.
Chalmers-Redman RM, Fraser AD, Ju WY, Wadia J, Tatton NA, Tatton WG. Mechanisms of nerve cell death: apoptosis or necrosis after cerebral ischaemia. Int Rev Neurobiol 1997;40:1-25. http://dx.doi.org/10.1016/S0074-7742(08)60713-8.
Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995;15(4):961-73.
http://dx.doi.org/10.1016/0896-6273(95)90186-8.
Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, et al. Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg 2013;116(4):869-80. http://dx.doi.org/10.1213/ANE.0b013e3182860fc9.
Zhang Q, Shao Y, Zhao C, Cai J, Sun S. N-methyl-D-aspartate receptor antagonist MK-801 prevents apoptosis in rats that have undergone fetal spinal cord transplantation following spinal hemisection. Exp Ther Med 2014;8(6):1731-6.
http://dx.doi.org/10.3892/etm.2014.2029.
Migita H, Kominami K, Higashida M, Maruyama R, Tuchida N, McDonald F, et al. Activation of adenosine A1 receptor-induced neural stem cell proliferation via MEK/ERK and Akt signaling pathways. J Neurosci Res 2008;86(13):2820-8.
http://dx.doi.org/10.1002/jnr.21742.
Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell stem cell 2011;8(1):59-71. http://dx.doi.org/10.1016/j.stem.2010.11.028.
Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A 2006;103(1):111-6.
http://dx.doi.org/10.1073/pnas.0509939103.
Jung SR, Kuok IT, Couron D, Rizzo N, Margineantu DH, Hockenbery DM, et al. Reduced cytochrome C is an essential regulator of sustained insulin secretion by pancreatic islets. J Biol Chem 2011;286(20):17422-34.