Efficacy of a bundle approach in preventing the incidence of ventilator associated pneumonia (VAP)
DOI:
https://doi.org/10.17305/bjbms.2017.2278Keywords:
Ventilator-associated pneumonia, VAP, primary prevention, epidemiology, medical devices, intratracheal intubation, bundleAbstract
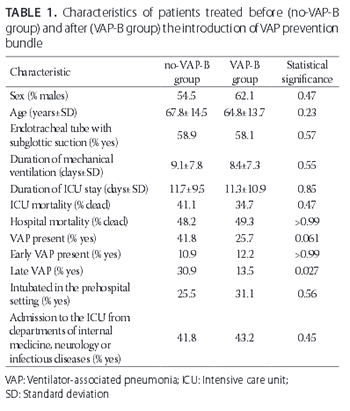
Ventilator-associated pneumonia (VAP) is a potentially preventable iatrogenic illness that may develop following mechanical ventilation. A bundle for the prevention of VAP consists of different measures which may vary between institutions, and may include: elevation of the head of the bed, oral care with chlorhexidine, subglottic suctioning, daily assessment for extubation and the need for proton-pump inhibitors, use of closed suction systems, and maintaining endotracheal cuff pressure at 25 cmH2O. Our aim was to determine the efficacy of a VAP prevention bundle, consisting of the above-mentioned measures, by evaluating the incidence of VAP before (no-VAP-B group) and after (VAP-B group) the introduction of the bundle. We retrospectively evaluated the data for patients who were mechanically ventilated with an endotracheal tube, in the period between 1 September and 31 December 2014 (no-VAP-B group, n = 55, 54.5% males, mean age 67.8 ± 14.5 years) and between 1 January to 30 April 2015 (VAP-B group, n = 74, 62.1% males, mean age 64.8 ± 13.7 years). There were no statistically significant differences between no-VAP-B and VAP-B groups in demographic data, intensive care unit (ICU) mortality, hospital mortality, duration of ICU treatment, and duration of mechanical ventilation. No significant differences in the rates of VAP and early VAP (onset ≤7 days after intubation) were found between no-VAP-B and VAP-B groups (41.8% versus 25.7%, p = 0.06 and 10.9% versus 12.2%, p > 0.99, respectively). However, a significant decrease in the late VAP (onset >8 days after intubation) was found in VAP-B group compared to no-VAP-B group (13.5% versus 30.9%, p = 0.027). Overall, our results support the use of VAP prevention bundle in clinical practice.
Downloads
References
Blot SI, Poelaert J, Kollef M. How to avoid microaspiration? A key element for the prevention of ventilator-associated pneumonia in intubated ICU patients. BMC Infect Dis 2014;14:119. https://doi.org/10.1186/1471-2334-14-119.
Kalanuria AA, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care 2014;18(2):208. https://doi.org/10.1186/cc13775.
Marini AL, Khan R, Mundekkadan S. Multifaceted bundle interventions shown effective in reducing VAP rates in our multidisciplinary ICUs. BMJ Open Quality 2016;5(1):u205566.w2278. DOI: 10.1136/bmjquality.u205566.w2278.
Russell CJ, Shiroishi MS, Siantz E, Wu BW, Patino CM. The use of inhaled antibiotic therapy in the treatment of ventilator-associated pneumonia and tracheobronchitis: A systematic review. BMC Pulm Med 2016;16:40. https://doi.org/10.1186/s12890-016-0202-8.
Coffin SE, Klompas M, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol 2008;29(Suppl 1):S31-40. DOI: 10.1086/591062.
Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol 2012;33(3):250-6. https://doi.org/10.1086/664049.
Wong T, Schlichting AB, Stoltze AJ, Fuller BM, Peacock A, Harland KK, et al. No decrease in early ventilator-associated pneumonia after early use of chlorhexidine. Am J Crit Care 2016;25(2):173-7. DOI: 10.4037/ajcc2016823.
Daniel M, Booth M, Ellis K, Maher S, Longmate A. Details behind the dots: How different intensive care units used common and contrasting methods to prevent ventilator associated pneumonia. BMJ Open Quality 2015;4(1):u207660.w3069. DOI: 10.1136/bmjquality.u207660.w3069.
Rodriguez FB, Monteiro CA, Lain JI, Guimaraes BL, Soares LS, Menezes WB, et al. A three-step approach to reduce ventilator-associated pneumonia. Crit Care 2009;13(Suppl 3):P25. DOI: 10.1186/cc7827.
Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165(7):867-903. https://doi.org/10.1164/ajrccm.165.7.2105078.
Klompas M, Yokoe DS, Weinstein RA. Automated surveillance of health care-associated infections. Clin Infect Dis 2009;48(9):1268-75. https://doi.org/10.1086/597591.