Proteasome inhibitors: new class of antitumor agents
DOI:
https://doi.org/10.17305/bjbms.2003.3484Keywords:
proteasome inhibitors, antitumor agentsAbstract
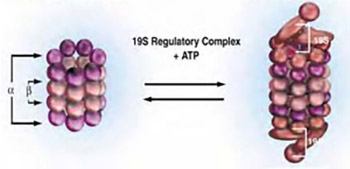
The ubiquitin-proteasome pathway is the principal pathway for intracellular protein degradation1,2 (Fig 1). This pathway selectively degrades an extensive number of short-lived regulatory proteins involved in the control of normal cellular processes. In order to be degraded, proteins targeted by the ubiquitin-proteasome pathway are covalently tagged by polyubiquitination, via a three-step enzymatic process, which ultimately leads to their recognition and degradation, by the 26S proteasome in a highly specific and regulated manner. This process is accomplished by the sequential action of three enzymes: an ATP-dependent ubiquitin-activating enzyme (E1), an ubiquitin-conjugating enzyme (E2) and an ubiquitin-pro-tein ligase (E3).3 This cascade covalently links the C terminus of ubiquitin to a free amino group on the target protein, usually the ε-amino of a lysine residue.
Downloads