Possible association of ABCB1:c.3435T>C polymorphism with high-density-lipoprotein-cholesterol response to statin treatment - a pilot study.
DOI:
https://doi.org/10.17305/bjbms.2014.3.43Keywords:
ABCB1 transporter, HMG-CoA reductase inhibitors, P-glycoproteinAbstract
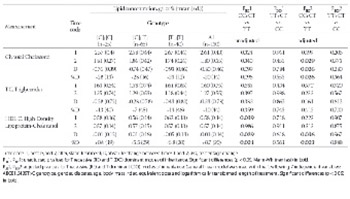
The gene product ABCB1 (formerly MDR1 or P-glycoprotein) is hypothesized to be involved in cholesterol cellular trafficking, redistribution and intestinal re-absorption. Carriers of the ABCB1:3435T allele have previously been associated with decreases in ABCB1 mRNA and protein concentrations and have been correlated with changes in serum lipid concentrations. The aim of this study was to investigate possible association between the ABCB1:3435T>C polymorphism and changes in lipids in patients following statin treatment. Outpatients (n=130) were examined: 43 men (33%), 87 women (67%): treated with atorvastatin or simvastatin (all patients with equivalent dose of 20 or 40 mg/d simvastatin). Blood was taken for ABCB1:3435T>C genotyping, and before and after statin treatment for lipid concentration determination (total cholesterol, high-density-lipoprotein-cholesterol (HDL-C), triglycerides). Change (Δ) in lipid parameters, calculated as differences between measurements before and after treatment, were analyzed with multiple regression adjustments: gender, diabetes, age, body mass index, equivalent statin dose, length of treatment. Univariate and multivariate analyses showed significant differences in ΔHDL-C (univariate p=0.029; multivariate p=0.036) and %ΔHDL-C (univariate p=0.021; multivariate p=0.023) between patients with TT (-0.05 ± 0.13 g/l; -6.8% ± 20%; respectively) and CC+CT genotypes (0.004 ± 0.15 g/l; 4.1 ± 26%; respectively). Reduction of HDL-C in homozygous ABCB1:3435TT patients suggests this genotype could be associated with a reduction in the benefits of statin treatment.
Downloads
References
Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158.
Sever PS, Poulter NR, Dahlöf B, Wedel H, Collins R, Beevers G, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial–lipid-lowering arm (ASCOT-LLA). Diabetes Care. 2005;28:1151–1157.
Gotto AM Jr. Review of primary and secondary prevention trials with lovastatin, pravastatin, and simvastatin. Am J Cardiol. 2005;96:34F–38F.
Willey VJ, Reinhold JA, Willey KH, Kelly BL, Cziraky MJ. Clinical and economic outcomes in patients switched to simvastatin in a community-based family medicine practice. Int J Clin Pract. 2010;64:1235–1238.
Barter PJ, O’Brien RC. Achievement of target plasma cholesterol levels in hypercholesterolaemic patients being treated in general practice. Atherosclerosis. 2000;149:199–205.
Natarajan N, Putnam RW, Yip AM, Frail D. Family practice patients’ adherence to statin medications. Can Fam Physician. 2007;53:2144–2145.
Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348:1442–1448.
Fellay J, Marzolini C, Meaden ER, Back DJ, Buclin T, Chave JP, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30–36.
Kurata Y, Ieiri I, Kimura M, Morita T, Irie S, Urae A, et al. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin Pharmacol Ther. 2002;72:209–219.
Bialecka M, Hnatyszyn G, Bielicka-Cymerman J, Drozdzik M. Znaczenie polimorfizmu genu MDR1 w patogenezie i leczeniu padaczki lekoopornej. Neurologia i Neurochirurgia Polska. 2005;39:476–481.
Tanigawara Y. Role of P-glycoprotein in drug disposition. Ther Drug Monit. 2000;22:137–140.
Garrigues A, Escargueil AE, Orlowski S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc Natl Acad Sci U S A. 2002;99:10347–10352.
Tous M, Ribas V, Ferré N, Escol?-Gil JC, Blanco-Vaca F, Alonso-Villaverde C, et al. Turpentine-induced inflammation reduces the hepatic expression of the multiple drug resistance gene, the plasma cholesterol concentration and the development of atherosclerosis in apolipoprotein E deficient mice. Biochim Biophys Acta. 2005;1733:192–198.
Jeannesson E, Siest G, Bastien B, Albertini L, Aslanidis C, Schmitz G, et al. Association of ABCB1 gene polymorphisms with plasma lipid and apolipoprotein concentrations in the STANISLAS cohort. Clin Chim Acta. 2009;403:198–202.
Rebecchi IMM, Rodrigues AC, Arazi SS, Genvigir FDV, Willrich MAV, Hirata MH, et al. ABCB1 and ABCC1 expression in peripheral mononuclear cells is influenced by gene polymorphisms and atorvastatin treatment. Biochem Pharmacol. 2009;77:66–75.
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97:3473–3478.
Wang D, Johnson AD, Papp AC, Kroetz DL, Sadée W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics. 2005;15:693–704.
Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528.
Markova S, Nakamura T, Sakaeda T, Makimoto H, Uchiyama H, Okamura N, et al. Genotype-dependent down-regulation of gene expression and function of MDR1 in human peripheral blood mononuclear cells under acute inflammation. Drug Metab Pharmacokinet. 2006;21:194–200.
Bonhomme-Faivre L, Devocelle A, Saliba F, Chatled S, Maccario J, Farinotti R, et al. MDR-1 C3435T polymorphism influences cyclosporine a dose requirement in liver-transplant recipients. Transplantation. 2004;78:21–25.
Ni LN, Li JY, Miao KR, Qiao C, Zhang SJ, Qiu HR, et al. Multidrug resistance gene (MDR1) polymorphisms correlate with imatinib response in chronic myeloid leukemia. Med Oncol. 2011;28:265–269.
Leschziner GD, Andrew T, Pirmohamed M, Johnson MR. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 2007;7:154–179.
Hung CC, Tai JJ, Lin CJ, Lee MJ, Liou HH. Complex haplotypic effects of the ABCB1 gene on epilepsy treatment response. Pharmacogenomics. 2005;6:411–417.
Keskitalo JE, Kurkinen KJ, Neuvoneni PJ, Niemi M. ABCB1 haplotypes differentially affect the pharmacokinetics of the acid and lactone forms of simvastatin and atorvastatin. Clin Pharmacol Ther. 2008;84:457–461.
Shou W, Wang D, Zhang K, Wang B, Wang Z, Shi J, et al. Gene-wide characterization of common quantitative trait loci for ABCB1 mRNA expression in normal liver tissues in the Chinese population. PLoS One. 2012;7:e46295.
Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta. 2009;1794:860–871.
Becker ML, Visser LE, van Schaik RHN, Hofman A, Uitterlinden AG, Stricker BHC. Common genetic variation in the ABCB1 gene is associated with the cholesterol-lowering effect of simvastatin in males. Pharmacogenomics. 2009;10:1743–1751.
Rodrigues AC, Rebecchi IMM, Bertolami MC, Faludi AA, Hirata MH, Hirata RDC. High baseline serum total and LDL cholesterol levels are associated with MDR1 haplotypes in Brazilian hypercholesterolemic individuals of European descent. Braz J Med Biol Res. 2005;38:1389–1397.
Kajinami K, Brousseau ME, Ordovas JM, Schaefer EJ. Polymorphisms in the multidrug resistance-1 (MDR1) gene influence the response to atorvastatin treatment in a gender-specific manner. Am J Cardiol. 2004;93:1046–1050.
Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol. 1998;81:582–587.
Fukuyama N, Homma K, Wakana N, Kudo K, Suyama A, Ohazama H, et al. Validation of the Friedewald Equation for Evaluation of Plasma LDL-Cholesterol. J Clin Biochem Nutr. 2008;43:1–5.
Charlton-Menys V, Betteridge DJ, Colhoun H, Fuller J, France M, Hitman GA, et al. Targets of statin therapy: LDL cholesterol, non-HDL cholesterol, and apolipoprotein B in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Clin Chem. 2009;55:473–480.
McTaggart F, Jones P. Effects of statins on high-density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther. 2008;22:321–338.
Ballantyne CM, Blazing MA, Hunninghake DB, Davidson MH, Yuan Z, DeLucca P, et al. Effect on high-density lipoprotein cholesterol of maximum dose simvastatin and atorvastatin in patients with hypercholesterolemia: results of the Comparative HDL Efficacy and Safety Study (CHESS). Am Heart J. 2003;146:862–869.
Mizutani T, Masuda M, Nakai E, Furumiya K, Togawa H, Nakamura Y, et al. Genuine functions of P-glycoprotein (ABCB1). Curr Drug Metab. 2008;9:167–174.
Hirai T, Fukui Y, Motojima K. PPARalpha agonists positively and negatively regulate the expression of several nutrient/drug transporters in mouse small intestine. Biol Pharm Bull. 2007;30:2185–2190.
Munshi A. Genetic variation in MDR1, LPL and eNOS genes and the response to atorvastatin treatment in ischemic stroke. Hum Genet. 2012;131:1775–1781.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2015 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.













