Ketamine exerts a protective role in a cell-based model of major depressive disorder via the inhibition of apoptosis and inflammation and activation of the Krebs cycle
DOI:
https://doi.org/10.17305/bjbms.2019.4222Keywords:
Ketamine, major depressive disorder, MDD, apoptosis, Krebs cycle, corticosterone, nuclear factor-κB, NF-κB, inflammation, cytokinesAbstract
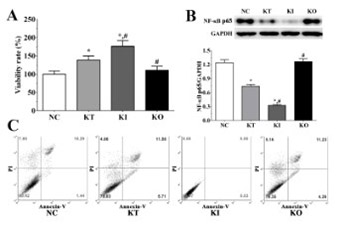
Major depressive disorder (MDD) is one of the most common psychiatric disorders characterized by major depressive episodes. Although great efforts have been made to develop antidepressant drugs that target the monoaminergic system, these drugs are effective in only approximately 50% of MDD patients. In this study, we established a model of depression in PC12 cells using corticosterone to investigate the effect of ketamine and nuclear factor-κB (NF-κB) on the cell viability, apoptosis, levels of pro-inflammatory cytokines, apoptosis-related molecules, and enzymes of the Krebs cycle. PC12 cells were divided into control (no treatment, NC), ketamine treatment (KT), ketamine treatment with the inhibition of NF-κB (KI), and ketamine treatment with the overexpression of NF-κB (KO) group. Blood serum samples were collected from patients with MDD (n = 10) and healthy controls (n = 10) between 2015 and 2017. Ketamine significantly increased the viability and decreased the apoptosis of PC12 cells in KT and KI vs. NC group, but not in KO group. The levels of anti-apoptotic molecules and Krebs cycle enzymes were significantly increased in KI vs. KT group, while the levels of pro-apoptotic molecules and pro-inflammatory cytokines were decreased in KI vs. KT group. In addition, the levels of pro-inflammatory cytokines in the serum of MDD patients were significantly increased. The antidepressant effect of ketamine was enhanced in KI and reduced in KO group. Our results indicate that ketamine exerts its antidepressant effect via the inhibition of apoptosis and inflammation and the activation of the Krebs cycle in PC12 cells. NF-κB might be a potential therapeutic target in MDD.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2019-05-13
Published 2020-02-05









