Making sense of subclinical cardiac alterations in patients with diabetes
DOI:
https://doi.org/10.17305/bjbms.2019.4349Keywords:
Body mass index, diabetes mellitus type 1, echocardiography, diastolic heart failure, left ventricular hypertrophyAbstract
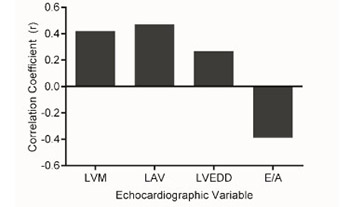
Patients with diabetes are prone to develop a distinct primary myocardial condition, diabetic cardiomyopathy, placing them at an increased risk for heart failure [1-3]. This occurs independently of hypertension, coronary artery disease, and other established causes of heart failure. Pertinent findings include increased mass, concentric changes, and diastolic dysfunction of the left ventricle [4,5]. Such adverse remodeling is common among patients with diabetes and appears to be strongly associated with its duration, suggesting a role for persistent metabolic stress [6-8]. However, which exact components of the diabetic syndrome determine these cardiac alterations is not clear. Moreover, most studies have investigated patients with type 2 diabetes, and it is uncertain whether patients with type 1 diabetes experience similar myocardial changes.
Continue reading the full text in the PDF version.
Citations
Downloads