Clear cell urothelial carcinoma of the urinary bladder - a rare pathological entity. A case report and a systematic review of the literature
DOI:
https://doi.org/10.17305/bjbms.2019.4182Keywords:
clear cell urothelial carcinoma, urinary bladder, reviewAbstract
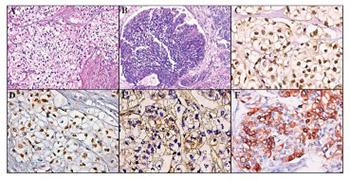
The most common histological type of urinary bladder cancer is urothelial carcinoma (UC). In contrast, the clear cell variant of urothelial carcinoma (CCUC) is quite a rare neoplasm. In this study, we report a case of an 81-year-old male, presenting with gross hematuria and acute urinary retention, which was subsequently diagnosed with CCUC at our pathology department. Furthermore, we provide a short systematic review of the literature (PubMed, Scopus, and Science Citation Index) for this rare histopathological entity and a brief discussion about its morphological and immunohistochemical (IHC) characteristics.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2019-03-23
Published 2019-11-08









