Inhibition of miR-9 decreases osteosarcoma cell proliferation
DOI:
https://doi.org/10.17305/bjbms.2019.4434Keywords:
Osteosarcoma, cell proliferation, metastasis, miR-9, E-cadherin, CDH1, matrix metalloproteinase 13, MMP-13, forkhead box O3, FOXO3a, Bcl-2-like protein 11, BCL2L11, β-catenin, CTNNB1Abstract
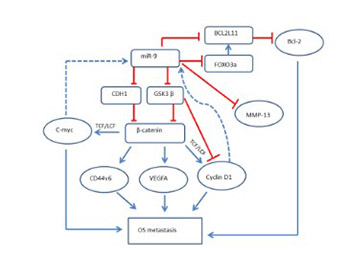
Osteosarcoma (OS) is the most common primary bone tumor that affects adolescents and young adults. Disruption of microRNA (miRNA) regulation is well established in the pathophysiology of different cancers, including OS. Increased expression of miR-9 in OS positively correlates with the tumor size, clinical stage, and distant metastasis. In the present study, we used two different OS cell lines, MG-63 and Saos-2, as in vitro models. miR-9 inhibitor and miR-9 mimics were used to study the function of miR-9 in these two cell lines. We determined the effect of miR-9 inhibition on cell proliferation, cell cycle, apoptosis, and the protein expression of different genes. Our results demonstrated that miR-9 inhibition in the human OS cell lines suppresses their metastatic potential, as determined by decreased cell proliferation and cell cycle arrest, decreased invasion, and increased apoptosis. The Western blot analysis showed that E-cadherin, matrix metalloproteinase 13, forkhead box O3, Bcl-2-like protein 11, and β-catenin are involved in miR-9 signaling. Moreover, miR-9 mimics rescued the effects caused by the inhibition of miR-9 in the OS cell lines. Our findings suggest that miR-9 is important for mediating OS cell migration, invasion, metastasis, and apoptosis. Inhibition of miR-9 could be further explored as a therapeutic target to treat OS.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2019-11-12
Published 2020-05-01









