Interventions of a clinical pharmacist in a medical intensive care unit – A retrospective analysis
DOI:
https://doi.org/10.17305/bjbms.2020.4612Keywords:
Clinical pharmacy, intensive care, interventions, medication errors, multidisciplinary care, SloveniaAbstract
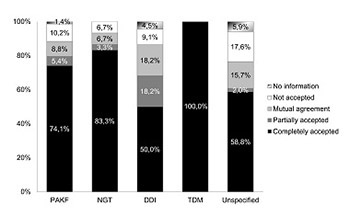
Several studies demonstrated a significant decrease in prescription errors, adverse drug events, treatment costs and improved patient outcomes, when a clinical pharmacist (CP) was a full member of a multidisciplinary team in the intensive care unit (ICU). Our aim was to evaluate the activities of a CP, included in a 12-bed medical ICU team of a university hospital in the course of several months. We conducted a retrospective analysis of all the CP’s interventions from March 2017 to November 2017, carried out and documented after reviewing and discussing patients’ medical data with the treating ICU physicians. We identified four main categories of CP’s interventions: pharmacotherapy adjustments to kidney function (PAKF category), drug-drug interactions (DDIs category), therapeutic monitoring of drugs with narrow therapeutic index (TDM category), and drug administration by the nasogastric tube (NGT category). During the study period, 533 patients were admitted to the medical ICU. The CP reviewed the medical data of 321 patients and suggested 307 interventions in 95 patients. There were 147 interventions of the PAKF category, 57 interventions of the TDM category, 30 interventions of the NGT category, and 22 interventions of the DDIs category. Fifty-one interventions were unspecified. The majority of all interventions (203/307) were related to antimicrobial drugs. ICU physicians completely accepted 80.2% of the CP’s suggestions. We observed that regular participation of the CP in the medical ICU team contributed to more individualized and improved pharmacological treatment of patients. Therefore, ICU teams should be encouraged to include CPs as regular team members.
Downloads