Comparison of three different regimens against Helicobacter pylori as a first-line treatment: A randomized clinical trial
DOI:
https://doi.org/10.17305/bjbms.2016.660Keywords:
Helicobacter pylori, quadruple therapy, sequential therapy, concomitant therapyAbstract
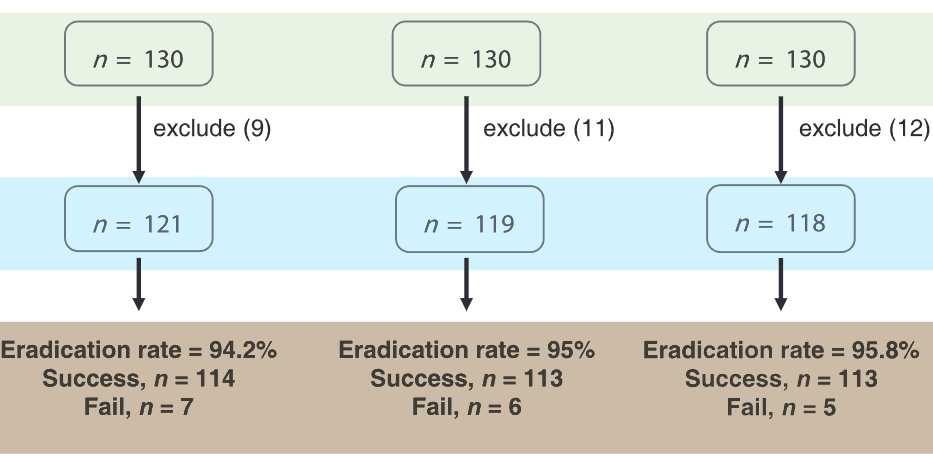
Treatments with bismuth-containing quadruple therapy (QT), sequential therapy (ST), or concomitant therapy (CT) have been proposed as empirical first-line regimens for Helicobacter pylori. We compared the efficacy and tolerability of 10 days bismuth-containing quadruple QT, 10 days ST, and 10 days CT with as first-line treatments for H. pylori in a randomized crossover study. The subjects were randomly divided into three groups. The first 130 patients were treated with rabeprazole, bismuth potassium citrate, metronidazole, and tetracycline for 10 days. The second 130 patients in the sequential group were treated with rabeprazole and amoxicillin for 5 days, and then rabeprazole, clarithromycin, and metronidazole for an additional 5 days. The last 130 patients in the concomitant group were treated with rabeprazole, amoxicillin, clarithromycin, and metronidazole for 10 days. H. pylori eradication was confirmed by urea breath test at 6 weeks. The primary outcome was eradication rates of first-line treatment by intention to treat and per protocol (PP) analyzes. There was no difference between the average ages and the male/female ratio of the groups. The PP analysis was performed on 121, 119, and 118 patients in the QT, ST, and CT groups, respectively. In the PP analysis, the successful eradication 94.2% (114/121), 95.0% (113/119), and 95.8% (113/118) the QT, ST, and CT groups, respectively. There was no significant difference among the three groups (p = 0.86). 10 days QT, ST, and CT are highly effective as empirical first-line therapies for H. pylori in the region with high clarithromycin resistance.
Citations
Downloads
References
Cogo LL, Monteiro CL, Miguel MD, Miguel OG, Cunico MM, Ribeiro ML, et al. Anti-Helicobacter pylori activity of plant extracts traditionally used for the treatment of gastrointestinal disorders. Braz J Microbiol 2010;41(2):304-9. http://dx.doi.org/10.1590/S1517-83822010000200007.
McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362(17):1597-604. http://dx.doi.org/10.1056/NEJMcp1001110.
Hentschel E, Brandstätter G, Dragosics B, Hirschl AM, Nemec H, Schütze K, et al. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med 1993;328(5):308-12. http://dx.doi.org/10.1056/NEJM199302043280503.
Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007;102(8):1808-25. http://dx.doi.org/10.1111/j.1572-0241.2007.01393.x.
Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: The Maastricht III Consensus Report. Gut 2007;56(6):772-81. http://dx.doi.org/10.1136/gut.2006.101634.
Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol 2009;24(10):1587-600. http://dx.doi.org/10.1111/j.1440-1746.2009.05982.x.
Chung JW, Lee GH, Han JH, Jeong JY, Choi KS, Kim do H, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology 2011;58(105):246-50.
Coelho LG, Maguinilk I, Zaterka S, Parente JM, do Carmo Friche Passos M, Moraes-Filho JP. 3rd Brazilian Consensus on Helicobacter pylori. Arq Gastroenterol 2013;50(2):S0004-28032013005000113.
Jodlowski TZ, Lam S, Ashby CR Jr. Emerging therapies for the treatment of Helicobacter pylori infections. Ann Pharmacother 2008;42(11):1621-39. http://dx.doi.org/10.1345/aph.1L234.
Dib J Jr, Alvarez B, Mendez L, Cruz ME. Efficacy of PPI, levofloxacin and amoxicillin in the eradication of Helicobacter pylori compared to conventional triple therapy at a Venezuelan hospital. Arab J Gastroenterol 2013;14(3):123-5. http://dx.doi.org/10.1016/j.ajg.2013.09.001.
Cho DK, Park SY, Kee WJ, Lee JH, Ki HS, Yoon KW, et al. The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy. Korean J Gastroenterol 2010;55(6):368-75. http://dx.doi.org/10.4166/kjg.2010.55.6.368.
Nadir I, Yonem O, Ozin Y, Kilic ZM, Sezgin O. Comparison of two different treatment protocols in Helicobacter pylori eradication. South Med J 2011;104(2):102-5. http://dx.doi.org/10.1097/SMJ.0b013e318200c209.
Liang X, Xu X, Zheng Q, Zhang W, Sun Q, Liu W, et al. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol 2013;11(7):802-7.e1.
Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: Systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol 2009;104(12):3069-79. http://dx.doi.org/10.1038/ajg.2009.555.
Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: Four-drug, three-antibiotic, non-bismuth-containing "concomitant therapy" versus triple therapy for Helicobacter pylori eradication. Helicobacter 2009;14(2):109-18. http://dx.doi.org/10.1111/j.1523-5378.2009.00671.x.
Heo J, Jeon SW. Changes in the eradication rate of conventional triple therapy for Helicobacter pylori infection in Korea. Korean J Gastroenterol 2014;63(3):141-5.
Xu MH, Zhang GY, Li CJ. Efficacy of bismuth-based quadruple therapy as first-line treatment for Helicobacter pylori infection. Zhejiang Da Xue Xue Bao Yi Xue Ban 2011;40(3):327-31.
Sun Q, Liang X, Zheng Q, Liu W, Xiao S, Gu W, et al. High efficacy of 14-day triple therapy-based, bismuth-containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter 2010;15(3):233-8. http://dx.doi.org/10.1111/j.1523-5378.2010.00758.x.
Agel E, Durmaz B, Tevfik MR, Asgın R. The isolation rate and antibiotic resistant pattern of Helicobacter pylori in dyspeptic patients. Turk J Med Sci 2000;30:143-6.
Goral V, Fadile YZ, Gül K. Antibiotic resistance in Helicobacter pylori infection. Clin Gastroenterohepatol 2000;11:87-9.
Agah S, Shazad B, Abbaszadeh B. Comparison of azithromycin and metronidazole in a quadruple-therapy regimen for Helicobacter pylori eradication in dyspepsia. Saudi J Gastroenterol 2009;15(4):225-8. http://dx.doi.org/10.4103/1319-3767.56091.
Rogha M, Pourmoghaddas Z, Rezaee M, Shirneshan K, Shahi Z. Azithromycin effect on Helicobacter pylori eradication: Double blind randomized clinical trial. Hepatogastroenterology 2009;56(91-92):722-4.
Zullo A, De Francesco V, Scaccianoce G, Manes G, Efrati C, Hassan C, et al. Helicobacter pylori eradication with either quadruple regimen with lactoferrin or levofloxacin-based triple therapy: A multicentre study. Dig Liver Dis 2007;39(9):806-10. http://dx.doi.org/10.1016/j.dld.2007.05.021.
Zheng Q, Chen WJ, Lu H, Sun QJ, Xiao SD. Comparison of the efficacy of triple versus quadruple therapy on the eradication of Helicobacter pylori and antibiotic resistance. J Dig Dis 2010;11(5):313-8. http://dx.doi.org/10.1111/j.1751-2980.2010.00457.x.
Cengiz N, Uslu Y, Gök F, Anarat A. Acute renal failure after overdose of colloidal bismuth subcitrate. Pediatr Nephrol 2005;20(9):1355-8. http://dx.doi.org/10.1007/s00467-005-1993-7.
Huwez F, Pall A, Lyons D, Stewart MJ. Acute renal failure after overdose of colloidal bismuth subcitrate. Lancet 1992;340(8830):1298. http://dx.doi.org/10.1016/0140-6736(92)93005-8.
Noach LA, Eekhof JL, Bour LJ, Posthumus Meyjes FE, Tytgat GN, Ongerboer de Visser BW. Bismuth salts and neurotoxicity. A randomised, single-blind and controlled study. Hum Exp Toxicol 1995;14(4):349-55. http://dx.doi.org/10.1177/096032719501400405.
Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011;377(9769):905-13. http://dx.doi.org/10.1016/S0140-6736(11)60020-2.
Ford AC, Malfertheiner P, Giguere M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: Systematic review and meta-analysis. World J Gastroenterol 2008;14(48):7361-70. http://dx.doi.org/10.3748/wjg.14.7361.
Sirimontaporn N, Thong-Ngam D, Tumwasorn S, Mahachai V. Ten-day sequential therapy of Helicobacter pylori infection in Thailand. Am J Gastroenterol 2010;105(5):1071-5. http://dx.doi.org/10.1038/ajg.2009.708.
Gao XZ, Qiao XL, Song WC, Wang XF, Liu F. Standard triple, bismuth pectin quadruple and sequential therapies for Helicobacter pylori eradication. World J Gastroenterol 2010;16(34):4357-62. http://dx.doi.org/10.3748/wjg.v16.i34.4357.
Yakut M, Çinar K, Seven G, Bahar K, Özden A. Sequential therapy for Helicobacter pylori eradication. Turk J Gastroenterol 2010;21(3):206-11.
Demir M, Ataseven H. The effects of sequential treatment as a first-line therapy for Helicobacter pylori eradication. Turk J Med Sci 2011;41(3):427-33.
Sezgin O, Altintas E, Nayir E, Uçbilek E. A pilot study evaluating sequential administration of a PPI-amoxicillin followed by a PPI-metronidazole-tetracycline in Turkey. Helicobacter 2007;12(6):629-32. http://dx.doi.org/10.1111/j.1523-5378.2007.00547.x.
Uygun A, Kadayifci A, Yesilova Z, Safali M, Ilgan S, Karaeren N. Comparison of sequential and standard triple-drug regimen for Helicobacter pylori eradication: A 14-day, open-label, randomized, prospective, parallel-arm study in adult patients with nonulcer dyspepsia. Clin Ther 2008;30(3):528-34. http://dx.doi.org/10.1016/j.clinthera.2008.03.009.
McNicholl AG, Marin AC, Molina-Infante J, Castro M, Barrio J, Ducons J, et al. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut 2014;63(2):244-9.
Liu KS, Hung IF, Seto WK, Tong T, Hsu AS, Lam FY, et al. Ten day sequential versus 10 day modified bismuth quadruple therapy as empirical firstline and secondline treatment for Helicobacter pylori in Chinese patients: An open label, randomised, crossover trial. Gut 2014;63(9):1410-5. http://dx.doi.org/10.1136/gutjnl-2013-306120.
Webber MA, Piddock LJ. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 2003;51(1):9-11. http://dx.doi.org/10.1093/jac/dkg050.
Okada M, Nishimura H, Kawashima M, Okabe N, Maeda K, Seo M, et al. A new quadruple therapy for Helicobacter pylori: Influence of resistant strains on treatment outcome. Aliment Pharmacol Ther 1999;13(6):769-74. http://dx.doi.org/10.1046/j.1365-2036.1999.00551.x.
Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol 2010;8(1):36-41.e1.
Hsu PI, Wu DC, Chen WC, Tseng HH, Yu HC, Wang HM, et al. Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for Helicobacter pylori infection. Antimicrob Agents Chemother 2014;58(10):5936-42. http://dx.doi.org/10.1128/AAC.02922-14.
Dos Santos AA, Carvalho AA. Pharmacological therapy used in the elimination of Helicobacter pylori infection: A review. World J Gastroenterol 2015;21(1):139-54.
Ang TL, Wang L, Ang D, Chiam P, Fock KM, Teo EK. Is there still a role for empiric first-line triple therapy using proton pump inhibitor, amoxicillin and clarithromycin for Helicobacter pylori infection in Singapore? Results of a time trend analysis. J Dig Dis 2013;14(2):100-4. http://dx.doi.org/10.1111/1751-2980.12024.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2015 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2015-10-21
Published 2016-01-01









