Donor-derived cell-free DNA as a diagnostic marker for kidney-allograft rejection: A systematic review and meta-analysis
DOI:
https://doi.org/10.17305/bb.2024.10049Keywords:
Donor-derived cell-free DNA (dd-cfDNA), diagnosis, kidney, meta-analysis, rejectionAbstract
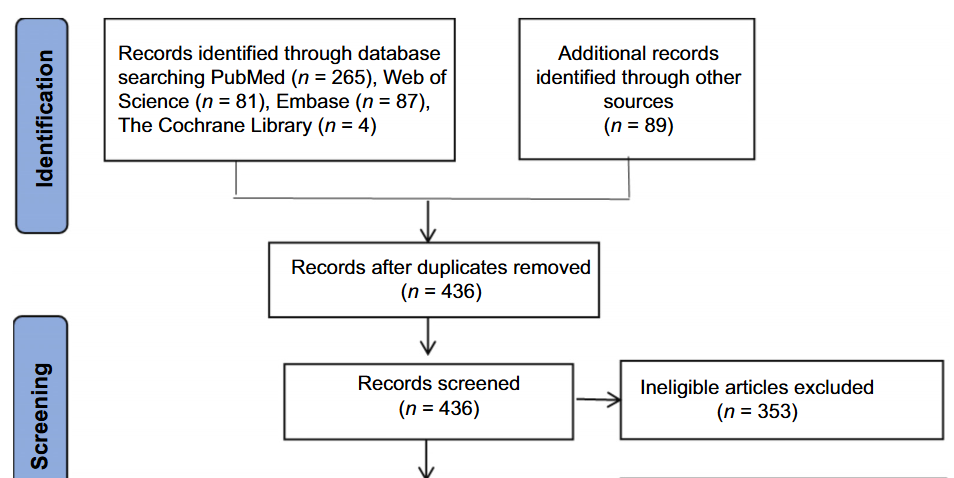
Donor-derived cell-free DNA (dd-cfDNA) has emerged as a promising biomarker for detecting graft rejection. This study aimed to evaluate the diagnostic accuracy and clinical value of applying it to kidney transplant rejection. Relevant literature on dd-cfDNA diagnostics in kidney transplant rejection was reviewed from PubMed, Embase, Cochrane Library, and Web of Science databases up to 2023. Data and study characteristics were extracted independently by two researchers, and disagreements were resolved through discussion. Diagnostic accuracy data for any rejection (AR) and antibody-mediated rejection (ABMR) were analyzed separately. Potential heterogeneity was analyzed by subgroup analysis or meta-regression. Funnel plots were used to clarify the presence or absence of publication bias. Nine publications provided data on dd-cfDNA accuracy in diagnosing patients with AR. The pooled sensitivity, specificity, and the area under the receiver operating characteristic (AUROC) curve with 95% confidence intervals (CI) were 0.59 (95% CI, 0.48-0.69), 0.83 (95% CI, 0.76-0.88), and 0.80 (95% CI, 0.76-0.83), respectively. Additionally, 12 studies focused on the diagnostic accuracy of dd-cfDNA for ABMR, showing pooled sensitivity, specificity, and the AUROC curve with 95% CI of 0.81 (95% CI, 0.72–0.88), 0.80 (95% CI, 0.73–0.86), and 0.87 (95% CI, 0.84-0.90), respectively. Study type, age group, and sample size contributed to heterogeneity. In summary, our findings indicate that while plasma dd-cfDNA accuracy in diagnosing patients with AR is limited by significant heterogeneity, it is a valuable biomarker for diagnosing ABMR.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2024 Yanbo Xing, Qiang Guo, Cong Wang; Haoying Shi, Jiarui Zheng, Yijun Jia; Chengyong Li, Chuan Hao

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2024-02-05
Published 2024-02-20









