SPDEF drives pancreatic adenocarcinoma progression via transcriptional upregulation of S100A16 and activation of the PI3K/AKT signaling pathway
DOI:
https://doi.org/10.17305/bb.2024.10346Keywords:
Pancreatic adenocarcinoma (PAAD), Sam's pointed domain-containing ETS transcription factor (SPDEF), S100A16, PI3K/AKT signaling pathwayAbstract
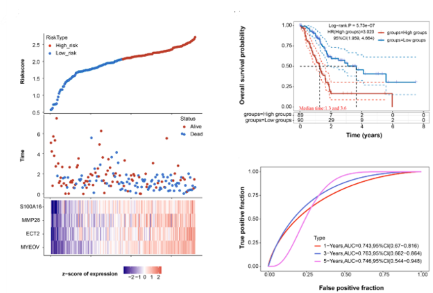
Pancreatic adenocarcinoma (PAAD) is a notably aggressive malignancy with limited treatment options and an unfavorable prognosis for patients. We aimed to investigate molecular mechanisms by which Sam's pointed domain-containing ETS transcription factor (SPDEF) exerts effects on PAAD progression. We analyzed differentially expressed genes (DEGs) and their integration with ETS family members using the The Cancer Genome Atlas (TCGA) database, hence identifying SPDEF as a core gene in PAAD. Kaplan-Meier survival analysis confirmed SPDEF’s prognostic potential. In vitro experiments validated the association with cell proliferation and apoptosis, affecting pancreatic cancer cell dynamics. We detected increased SPDEF expression in PAAD tumor samples. Our in vitro studies revealed that SPDEF regulates mRNA and protein expression levels, and significantly affects cell proliferation. Moreover, SPDEF was associated with reduced apoptosis and enhanced cell migration and invasion. In-depth analysis of SPDEF-targeted genes revealed four crucial genes for advanced prognostic model, among which S100A16 was significantly correlated with SPDEF. Mechanistic analysis showed that SPDEF enhances the transcription of S100A16, which in turn enhances PAAD cell migration, proliferation, and invasion by activating the PI3K/AKT signaling pathway. Our study revealed the critical role of SPDEF in promoting PAAD by upregulating S100A16 transcription and stimulating the PI3K/AKT signaling pathway. This knowledge deepened our understanding of pancreatic cancer's molecular progression and unveiled potential therapeutic strategies targeting SPDEF-driven pathways.
Citations
Downloads
Downloads
Published
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Issue
Section
Categories
License
Copyright (c) 2024 Hang Jiang, Zhiqian Xue, Liping Zhao, Boyuan Wang, Chenfei Wang, Haihan Song, Jianjun Sun

This work is licensed under a Creative Commons Attribution 4.0 International License.









