Impact of RANGAP1 SUMOylation on Smad4 nuclear export by bioinformatic analysis and cell assays
DOI:
https://doi.org/10.17305/bb.2024.10443Keywords:
Glioma, RANGAP1, TGF-β/Smad signaling pathway, SUMOylation, SUMO1Abstract
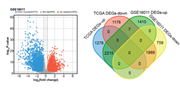
Small Ubiquitin-like Modifier (SUMOylation) regulates a variety of cellular activities, and its dysregulation has been associated with glioma etiology. The aim of this research was to clarify the function of SUMOylation-related genes in glioma and determine relevant prognostic markers. The Cancer Genome Atlas (TCGA) Glioma and GSE16011 datasets were analyzed through bioinformatics to identify Ran GTPase activating protein 1 (RANGAP1) as the hub gene for further study. Experimental validation consisted of quantitative real-time polymerase chain reaction (qRT-PCR), western blotting (WB), and immunoprecipitation (IP) to evaluate RANGAP1 expression, function, and interaction with SUMO1. To assess the role of RANGAP1 knockdown and SUMOylation in glioma cells, various assays were conducted, including cell proliferation, migration, invasion, and apoptosis. In addition, cell cycle analysis and immunofluorescence were performed. Through bioinformatics, RANGAP1 was identified as a crucial prognostic gene for glioma. Experimental studies confirmed the downregulation of RANGAP1 in glioma cells and verified that RANGAP1 repair impedes tumor growth. When it comes to RANGAP1 silencing, it enhanced cell proliferation, invasion and migration. Additionally, SUMO1 was identified as a specific SUMO molecule coupled to RANGAP1, affecting the location of Sma and Mad related protein 4 (Smad4) in the nucleocytoplasm and the transforming growth factor (TGF)-β/Smad signaling pathway. The functional impact of RANGAP1 SUMOylation on cell proliferation and migration was further confirmed through experiments using a SUMOylation-impairing mutation (K524R). Our findings suggest that RANGAP1 may be a potential prognostic marker in gliomas and could play a role in regulating cell proliferation, migration, and invasion. SUMOylation of RANGAP1 is responsible for regulating the TGF-β/Smad signaling pathway, which is crucial for the progression of tumors. Further investigations and experiments are necessary to confirm these results.
Citations
Downloads
Downloads
Additional Files
Published
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
Issue
Section
Categories
License
Copyright (c) 2024 Feng Zhang, Jun Yang, Yifei Cheng

This work is licensed under a Creative Commons Attribution 4.0 International License.









