Glutathione protects against hepatic injury in a murine model of primary Sjögren’s syndrome
DOI:
https://doi.org/10.17305/bjbms.2016.1059Keywords:
Primary Sjögren’s syndrome, glutathione, hepatic injury, inflammation, oxidative stressAbstract
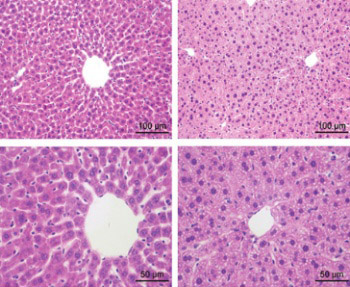
Primary Sjögren’s syndrome (pSS) is a systemic autoimmune disease which may cause complications such as hepatic dysfunction and injury. As an important antioxidant, reduced glutathione (GSH) has been reported protecting against hepatic injury induced by some diseases, but the role of GSH in pSS is poorly understood. This study aims at investigating the role of GSH in hepatic injury during pSS. A murine model of pSS, non-obese diabetic (NOD) mice, was used for GSH administration via tail intravenous injection. Enzyme-linked immunosorbent assay (ELISA) was performed to detect serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), as well as the levels of GSH, tumor necrosis factor, interleukin (IL) 10, integrin alpha M, IL1B, malondialdehyde, nicotinamide adenine dinucleotide phosphate oxidase 4, and superoxide dismutases in hepatocyte homogenates. Hematoxylin-eosin staining was performed to observe hepatic histology. The results showed that serum AST and ALT levels were up-regulated in the NOD mice (p = 0.0021 and 0.0048), but were significantly recovered after the GSH administration (p = 0.0081 and 0.0263). The NOD mice exhibited disturbed hepatic tissue structure, which was attenuated by GSH. The GSH administration could also promote the production of GSH in the hepatocytes (p = 0.0264), and control the levels of inflammatory factors and oxidative stress-related factors. These results indicate that GSH has significant effects on protecting against the hepatic injury during pSS, which may be associated with its regulation of the inflammatory factors and oxidative stress-related factors. This study suggests that GSH is a promising therapeutic strategy for controlling hepatic injury during pSS and offers valuable information for further research.
Citations
Downloads
References
Nikolov NP, Illei GG. Pathogenesis of Sjögren's syndrome. Curr Opin Rheumatol 2009;21(5):465-70. http://dx.doi.org/10.1097/BOR.0b013e32832eba21.
Arakaki R, Eguchi H, Yamada A, Kudo Y, Iwasa A, Enkhmaa T, et al. Anti-inflammatory effects of rebamipide eyedrop administration on ocular lesions in a murine model of primary Sjögren's syndrome. PLoS One 2014;9(5):e98390. http://dx.doi.org/10.1371/journal.pone.0098390.
Mori K, Iijima M, Koike H, Hattori N, Tanaka F, Watanabe H, et al. The wide spectrum of clinical manifestations in Sjögren's syndrome-associated neuropathy. Brain 2005;128:2518-34. http://dx.doi.org/10.1093/brain/awh605.
Pérez P, Anaya JM, Aguilera S, Urzúa U, Munroe D, Molina C, et al. Gene expression and chromosomal location for susceptibility to Sjögren's syndrome. J Autoimmun 2009;33(2):99-108. http://dx.doi.org/10.1016/j.jaut.2009.05.001.
Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren's syndrome: Findings in humans and mice. Arthritis Rheum 2008;58(3):734-43. http://dx.doi.org/10.1002/art.23214.
Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren's syndrome. Nat Genet 2013;45(11):1284-92. http://dx.doi.org/10.1038/ng.2792.
Mavragani CP, Nezos A, Moutsopoulos HM. New advances in the classification, pathogenesis and treatment of Sjogren's syndrome. Curr Opin Rheumatol 2013;25(5):623-9. http://dx.doi.org/10.1097/BOR.0b013e328363eaa5.
Zhang Z, Dong Y. Clinical manifestations and immunological features of primary Sjögren's syndrome with liver involvement: Analysis of thirty cases. Chin Med J (Engl) 1998;111(3):220-3.
Kita M, Eguchi K, Kawabe Y, Tsubio M, Kawakami A, Nakamura H, et al. Abnormal liver function in patients with Sjogren's syndrome. Acta Med Nagasaki 1996;41(3-4):31-7.
Lee SW, Kim BK, Park JY, Kim DY, Ahn SH, Song J, et al. Clinical predictors of silent but substantial liver fibrosis in primary Sjogren’s syndrome. Mod Rheumatol 2015;20:1-22.
Rajesh M, Pan H, Mukhopadhyay P, Batkai S, Osei-Hyiaman D, Hasko G, et al. Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol 2007;82(6):1382-9. http://dx.doi.org/10.1189/jlb.0307180.
Zhou Z, Sun X, James Kang Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med (Maywood) 2002;227(3):214-22.
Kono N, Inoue T, Yoshida Y, Sato H, Matsusue T, Itabe H, et al. Protection against oxidative stress-induced hepatic injury by intracellular Type II platelet-activating factor acetylhydrolase by metabolism of oxidized phospholipids in vivo. J Biol Chem 2008;283(3):1628-36. http://dx.doi.org/10.1074/jbc.M708622200.
Mizrahi S, Dolberg L, Jacobsohn WZ. Alteration in aminotransferase levels in rats after acute hepatic injury. Isr J Med Sci 1987;23(3):188-92.
Fu H, Chen H, Wang C, Xu H, Liu F, Guo M, et al. Flurbiprofen, a cyclooxygenase inhibitor, protects mice from hepatic ischemia/reperfusion injury by inhibiting GSK-3ß signaling and mitochondrial permeability transition. Mol Med 2012;18:1128-35. http://dx.doi.org/10.2119/molmed.2012.00088.
Golab F, Kadkhodaee M, Zahmatkesh M, Hedayati M, Arab H, Schuster R, et al. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int 2009;75(8):783-92. http://dx.doi.org/10.1038/ki.2008.683.
Wang J, Chen Y, Gao N, Wang Y, Tian Y, Wu J, et al. Inhibitory effect of glutathione on oxidative liver injury induced by dengue virus serotype 2 infections in mice. PLoS One 2013;8(1):e55407. http://dx.doi.org/10.1371/journal.pone.0055407.
Lee BH, Carcamo WC, Chiorini JA, Peck AB, Nguyen CQ. Gene therapy using IL-27 ameliorates Sjögren's syndrome-like autoimmune exocrinopathy. Arthritis Res Ther 2012;14(4):R172. http://dx.doi.org/10.1186/ar3925.
Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 2010;51(1):246-54. http://dx.doi.org/10.1002/hep.23267.
Chen M, Zhang J, Liu T, Zhou M, Xu G. Characteristic of hepatic lesion in primary sjogren syndrome (52 cases reports). China J Modern Med 2006;16(3):438-43 (in Chinese).
Skopouli FN, Barbatis C, Moutsopoulos HM. Liver involvement in primary Sjögren's syndrome. Br J Rheumatol 1994;33(8):745-8. http://dx.doi.org/10.1093/rheumatology/33.8.745.
Matsumoto T, Morizane T, Aoki Y, Yamasaki S, Nakajima M, Enomoto N, et al. Autoimmune hepatitis in primary Sjogren's syndrome: Pathological study of the livers and labial salivary glands in 17 patients with primary Sjogren's syndrome. Pathol Int 2005;55(2):70-6. http://dx.doi.org/10.1111/j.1440-1827.2005.01790.x.
Selmi C, Meroni PL, Gershwin ME. Primary biliary cirrhosis and Sjögren's syndrome: Autoimmune epithelitis. J Autoimmun 2012;39(1-2):34-42. http://dx.doi.org/10.1016/j.jaut.2011.11.005.
Qin B, Wang J, Liang Y, Yang Z, Zhong R. The association between TNF-a, IL-10 gene polymorphisms and primary Sjögren's syndrome: A meta-analysis and systemic review. PLoS One 2013;8(5):e63401. http://dx.doi.org/10.1371/journal.pone.0017631.
Willeke P, Schotte H, Schlüter B, Erren M, Becker H, Dyong A, et al. Interleukin 1beta and tumour necrosis factor alpha secreting cells are increased in the peripheral blood of patients with primary Sjögren's syndrome. Ann Rheum Dis 2003;62(4):359-62. http://dx.doi.org/10.1136/ard.62.4.359.
Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol 2009;36(1):4-12. http://dx.doi.org/10.1007/s12016-008-8091-0.
Cheng L, Wang J, Li X, Xing Q, Du P, Su L, et al. Interleukin-6 induces Gr-1+ CD11b+ myeloid cells to suppress CD8+ T cell-mediated liver injury in mice. PLoS One 2011;6(3):e17631.
Liang Q, Sheng Y, Jiang P, Ji L, Xia Y, Min Y, et al. The gender-dependent difference of liver GSH antioxidant system in mice and its influence on isoline-induced liver injury. Toxicology 2011;280(1-2):61-9. http://dx.doi.org/10.1016/j.tox.2010.11.010.
Videla LA. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J Hepatol 2009;1(1):72-8. http://dx.doi.org/10.4254/wjh.v1.i1.72.
Bettaieb A, Jiang JX, Sasaki Y, Chao TI, Kiss Z, Chen X, et al. Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology 2015;149(2):468-80. http://dx.doi.org/10.1053/j.gastro.2015.04.009.
Olsvik PA, Kristensen T, Waagbø R, Rosseland BO, Tollefsen KE, Baeverfjord G, et al. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comp Biochem Physiol C Toxicol Pharmacol 2005;141(3):314-23. http://dx.doi.org/10.1016/j.cbpc.2005.07.009.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-03-05
Published 2016-08-02









