UPP1 and AHSA1 as emerging biomarkers and targets in pancreatic cancer: A proteomic approach
DOI:
https://doi.org/10.17305/bb.2025.11958Keywords:
Pancreatic cancer, PC, proteomics, differentiated pancreatic cancer, target, experimental validationAbstract
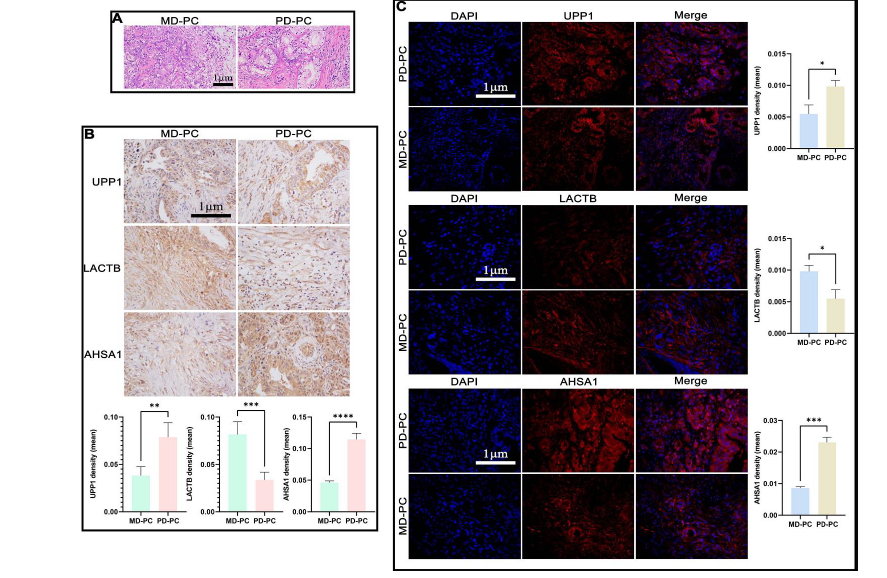
The specific protein targets involved in pancreatic cancer (PC) pathogenesis and its varying levels of differentiation remain incompletely understood. Advanced proteomic methodologies provide a powerful means of identifying key regulatory proteins and signaling pathways central to cancer progression. In this study, proteomic analyses were performed on PC tissue samples of different differentiation grades, along with adjacent non-cancerous (para-PC) tissues. Bioinformatics techniques were used to identify differentially expressed proteins (DEPs) and their associated pathways. Key target proteins were validated using the Gene Expression Profiling Interactive Analysis (GEPIA) database, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), Western blotting, immunohistochemistry (IHC), and immunofluorescence (IF). A total of 431 DEPs were identified between PC and para-PC tissues, while 470 DEPs distinguished poorly differentiated (PD) from moderately differentiated (MD) PCs. Functional enrichment analysis revealed that these DEPs participate in various biological processes and signaling pathways. Five DEPs were common to both comparisons, with Uridine Phosphorylase 1 (UPP1), Lactamase Beta, and Activator of HSP90 ATPase Activity 1 (AHSA1) showing particularly notable differences. UPP1 and AHSA1 were significantly upregulated in PC tissues relative to adjacent tissues and exhibited even higher expression in PD-PCs compared to MD ones. These findings were consistently supported by GEPIA, RT-qPCR, Western blotting, IHC, and IF analyses. This study identifies UPP1 and AHSA1 as key proteins linked to PC differentiation and progression, highlighting their potential as diagnostic markers and therapeutic targets. These insights enhance our understanding of the molecular mechanisms underlying PC and open new avenues for precision treatment strategies.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2025 Kongfan Zhu, Hua Hu, Yuanfa Tao, Zhijian Yang, Hanjun Li

This work is licensed under a Creative Commons Attribution 4.0 International License.









