Differential expression of androgen, estrogen, and progesterone receptors in benign prostatic hyperplasia
DOI:
https://doi.org/10.17305/bjbms.2016.1209Keywords:
Benign prostatic hyperplasia, androgen receptor, estrogen receptor α, estrogen receptor β, progesterone receptor, rat modelAbstract
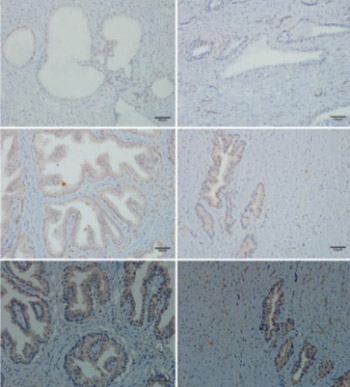
This study aimed to identify the differential expression levels of androgen receptor (AR), estrogen receptors (ERα, ERβ), and progesterone receptor (PGR) between normal prostate and benign prostatic hyperplasia (BPH). The combination of immunohistochemistry, quantitative real-time reverse transcription polymerase chain reaction, and Western blotting assay was used to identify the distribution and differential expression of these receptors at the immunoactive biomarker, transcriptional, and protein levels between 5 normal human prostate tissues and 40 BPH tissues. The results were then validated in a rat model of BPH induced by testosterone propionate and estradiol benzoate. In both human and rat prostate tissues, AR was localized mainly to epithelial and stromal cell nuclei; ERα was distributed mainly to stromal cells, but not exclusively; ERβ was interspersed in the basal layer of epithelium, but sporadically in epithelial and stromal cells; PGR was expressed abundantly in cytoplasm of epithelial and stromal cells. There were decreased expression of ERα and increased expression of PGR, but no difference in the expression of ERβ in the BPH compared to the normal prostate of both human and rat. Increased expression of AR in the BPH compared to the normal prostate of human was observed, however, the expression of AR in the rat prostate tissue was decreased. This study identified the activation of AR and PGR and repression of ERα in BPH, which indicate a promoting role of AR and PGR and an inhibitory role of ERα in the pathogenesis of BPH.
Citations
Downloads
References
. Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res. 2008;20 Suppl 3:S11-8.
http://dx.doi.org/10.1038/ijir.2008.55.
. Roehrborn CG. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med Clin North Am. 2011;95(1):87-100.
http://dx.doi.org/10.1016/j.mcna.2010.08.013.
. Lepor H. Pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. Rev Urol. 2004;6 Suppl 9:3-10.
. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29-41. http://dx.doi.org/10.1038/nrurol.2010.207.
. Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation. 2011;82(4-5):184-99.
http://dx.doi.org/10.1016/j.diff.2011.04.006.
. Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol. 2013;182(6):1942-9.
http://dx.doi.org/10.1016/j.ajpath.2013.02.028.
. Kawashima H, Nakatani T. Involvement of estrogen receptors in prostatic diseases. Int J Urol. 2012;19(6):512-22; author reply 522-3. http://dx.doi.org/10.1111/j.1442-2042.2012.02987.x.
. Yu Y, Liu L, Xie N, Xue H, Fazli L, Buttyan R, et al. Expression and function of the progesterone receptor in human prostate stroma provide novel insights to cell proliferation control. J Clin Endocrinol Metab. 2013;98(7):2887-96. http://dx.doi.org/10.1210/jc.2012-4000.
. National Advisory Committee for Laboratory Animal Research. Guidelines on the care and use of animals for scientific purposes. Singapore: National Advisory Committee for Laboratory Animal Research; 2004.
. George JN, Jonathan IE. Immunohistology of the prostate, bladder, kidney, and testis. In: David JD editor. Diagnostic Immunohistochemistry. 3rd ed. Ch. 16. Philadelphia: Saunders;2011. p. 593-618.
. Nicholson TM, Sehgal PD, Drew SA, Huang W, Ricke WA. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation. 2013;85(4-5):140-9.
http://dx.doi.org/10.1016/j.diff.2013.02.006.
. Royuela M, de Miguel MP, Bethencourt FR, Sánchez-Chapado M, Fraile B, Arenas MI, et al. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol. 2001;168(3):447-54.
http://dx.doi.org/10.1677/joe.0.1680447.
. Tsurusaki T, Aoki D, Kanetake H, Inoue S, Muramatsu M, Hishikawa Y, et al. Zone-dependent expression of estrogen receptors alpha and beta in human benign prostatic hyperplasia. J Clin Endocrinol Metab. 2003;88(3):1333-40.
http://dx.doi.org/10.1210/jc.2002-021015.
. Suzuki K, Matsui H, Hasumi M, Ono Y, Nakazato H, Koike H, et al. Gene expression profiles in human BPH: Utilization of laser-capture microdissection and quantitative real-time PCR. Anticancer Res. 2001;21(6A):3861-4.
. Shin IS, Lee MY, Ha HK, Seo CS, Shin HK. Inhibitory effect of Yukmijihwang-tang, a traditional herbal formula against testosterone-induced benign prostatic hyperplasia in rats. BMC Complement Altern Med. 2012;12:48.
http://dx.doi.org/10.1186/1472-6882-12-48.
. Scolnik MD, Servadio C, Abramovici A. Comparative study of experimentally induced benign and atypical hyperplasia in the ventral prostate of different rat strains. J Androl. 1994;15(4):287-97.
. Bostwick D. Pathology of benign prosatic hyperplasia. In: Kirby R, McConnell J, Fitzpatrick J, Roehrborn C, Boyle P, editors. Textbook of Benign Prostatic Hyperplasia. Arbington, UK: Taylor and Francis; 2005.
. Alonso-Magdalena P, Brössner C, Reiner A, Cheng G, Sugiyama N, Warner M, et al. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci U S A. 2009;106(8):2859-63.
http://dx.doi.org/10.1073/pnas.0812666106.
. Hetzl AC, Fávaro WJ, Billis A, Ferreira U, Cagnon VH. Steroid hormone receptors, matrix metalloproteinases, insulin-like growth factor, and dystroglycans interactions in prostatic diseases in the elderly men. Microsc Res Tech. 2012;75(9):1197-205. http://dx.doi.org/10.1002/jemt.22049.
. van der Sluis TM, Vis AN, van Moorselaar RJ, Bui HN, Blankenstein MA, Meuleman EJ, et al. Intraprostatic testosterone and dihydrotestosterone. Part I: Concentrations and methods of determination in men with benign prostatic hyperplasia and prostate cancer. BJU Int. 2012;109(2):176-82.
http://dx.doi.org/10.1111/j.1464-410X.2011.10651.x.
. Morales A. Monitoring androgen replacement therapy: Testosterone and prostate safety. J Endocrinol Invest. 2005;28(3 Suppl):122-7.
. Mahapokai W, Van Sluijs FJ, Schalken JA. Models for studying benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2000;3(1):28-33. http://dx.doi.org/10.1038/sj.pcan.4500391.
. Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci. 2009;1155:174-86. http://dx.doi.org/10.1111/j.1749-6632.2009.04360.x.
. McPherson SJ, Hussain S, Balanathan P, Hedwards SL, Niranjan B, Grant M, et al. Estrogen receptor-beta activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFalpha mediated. Proc Natl Acad Sci U S A. 2010;107(7):3123-8.
http://dx.doi.org/10.1073/pnas.0905524107.
. Rohrmann S, Nelson WG, Rifai N, Kanarek N, Basaria S, Tsilidis KK, et al. Serum sex steroid hormones and lower urinary tract symptoms in Third National Health and Nutrition Examination Survey (NHANES III). Urology. 2007;69(4):708-13. http://dx.doi.org/10.1016/j.urology.2007.01.011.
. Hammarsten J, Damber JE, Karlsson M, Knutson T, Ljunggren O, Ohlsson C, et al. Insulin and free oestradiol are independent risk factors for benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2009;12(2):160-5. http://dx.doi.org/10.1038/pcan.2008.50.
. Kristal AR, Schenk JM, Song Y, Arnold KB, Neuhouser ML, Goodman PJ, et al. Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: Results from the prostate cancer prevention trial. Am J Epidemiol. 2008;168(12):1416-24.
http://dx.doi.org/10.1093/aje/kwn272.
. Zhang P, Hu WL, Cheng B, Zeng YJ, Wang XH, Liu TZ, et al. Which play a more important role in the development of large-sized prostates (=80 ml), androgen receptors or estrogen receptors? A comparative study. Int Urol Nephrol. 2016;48(3):325-33.
http://dx.doi.org/10.1007/s11255-015-1181-z.
. Luetjens CM, Didolkar A, Kliesch S, Paulus W, Jeibmann A, Böcker W, et al. Tissue expression of the nuclear progesterone receptor in male non-human primates and men. J Endocrinol. 2006;189(3):529-39. http://dx.doi.org/10.1677/joe.1.06348.
. Mobbs BG, Johnson IE, DeSombre ER, Toth J, Hughes A. Regulation of estrogen and progestin receptor concentrations in an experimental rat prostatic carcinoma by estrogen, antiestrogen, and progesterone. Cancer Res. 1987;47(10):2645-51.
Downloads
Additional Files
Published
How to Cite
Accepted 2016-04-23
Published 2016-07-02









