Microsatellite instability and B-type Raf proto-oncogene mutation in colorectal cancer: Clinicopathological characteristics and effects on survival
DOI:
https://doi.org/10.17305/bjbms.2016.1238Keywords:
Microsatellite instability, DNA mismatch repair genes, B-type Raf proto-oncogene mutation, survival, polymerase chain reaction, immunohistochemistryAbstract
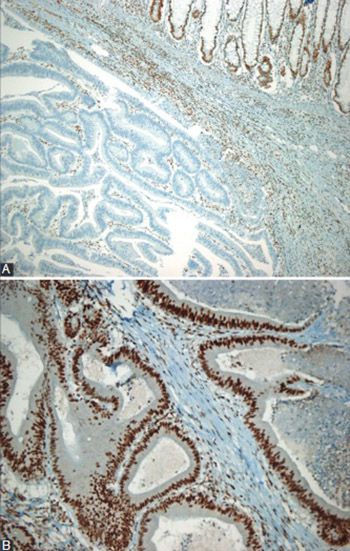
Prognostic significance of microsatellite instability (MSI) status and B-type Raf proto-oncogene (BRAF) mutation in colorectal cancer is controversial. The aim of this study was to examine the clinical and pathological characteristics associated with microsatellite stability and the effect of MSI and BRAF mutation on the survival of patients with colorectal cancer. The study included 145 colorectal cancer cases. All the patients were examined for DNA mismatch repair (MMR) proteins with an immunohistochemical method. Molecular assessment of MSI was available in a subset of 41 patients. In addition, BRAF mutation analysis was performed in 30 cases. Immunohistochemically, MMR deficiency was present in 28 (19.3%) patients. Female gender (p = 0.001), lesion size ≥5 cm (p = 0.013), Crohn-like response (p = 0.035), and right-sided localization (p < 0.001) were significantly more frequent among MMR-deficient patients. The overall survival was 44.1 ± 5.1 months (95% confidence interval [CI], 33.7-54.4). Multivariate analyses identified only high tumor grade as an independent predictor of poor overall survival: odd ratio, 6.7 (95% CI 2.1-21.7), p = 0.002. In the subset of patients with available BRAF assessment (n = 30), a negative BRAF status was associated with better survival when compared to a positive BRAF status (36.7 ± 2.1 vs. 34.1 ± 7.2 months, p = 0.048). The sensitivity and specificity of the immunohistochemical method in predicting positive MSI status, with the molecular method as a reference, were 85.7% (95% CI: 56.2%-97.5%) and 88.9% (95% CI: 69.7%-97.1%), respectively. BRAF appears to be a significant predictor of a worse outcome in patients with colorectal cancer. Further studies with a large spectrum of clinical and biological variables are warranted.
Citations
Downloads
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-917.
http://dx.doi.org/10.1002/ijc.25516.
Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1688-94.
http://dx.doi.org/10.1158/1055-9965.EPI-09-0090.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
http://dx.doi.org/10.3322/caac.21208.
Herzig DO, Tsikitis VL. Molecular markers for colon diagnosis, prognosis and targeted therapy. J Surg Oncol. 2015;111(1):96-102. http://dx.doi.org/10.1002/jso.23806.
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248-57.
Lynch HT, Smyrk T, Lynch JF. Molecular genetics and clinical-pathology features of hereditary nonpolyposis colorectal carcinoma (Lynch syndrome): historical journey from pedigree anecdote to molecular genetic confirmation. Oncology. 1998;55(2):103-8.
http://dx.doi.org/10.1159/000011843.
Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816-19. http://dx.doi.org/10.1126/science.8484122.
Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260(5109):812-16.
http://dx.doi.org/10.1126/science.8484121.
Merok MA, Ahlquist T, Royrvik EC, Tufteland KF, Hektoen M, Sjo OH, et al. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013;24(5):1274-82. http://dx.doi.org/10.1093/annonc/mds614.
Lothe RA, Peltomaki P, Meling GI, Aaltonen LA, Nystrom-Lahti M, Pylkkanen L, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53(24):5849-52.
Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609-18.
http://dx.doi.org/10.1200/JCO.2005.01.086.
Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46(15):2788-98.
http://dx.doi.org/10.1016/j.ejca.2010.05.009.
Benatti P, Gafa R, Barana D, Marino M, Scarselli A, Pedroni M, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005;11(23):8332-40.
http://dx.doi.org/10.1158/1078-0432.CCR-05-1030.
Malesci A, Laghi L, Bianchi P, Delconte G, Randolph A, Torri V, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13(13):3831-39.
http://dx.doi.org/10.1158/1078-0432.CCR-07-0366.
Samowitz WS, Curtin K, Ma KN, Schaffer D, Coleman LW, Leppert M, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10(9):917-23.
Gatalica Z, Vranic S, Xiu J, Swensen J, Reddy S. High microsatellite instability (MSI-H) colorectal carcinoma: a brief review of predictive biomarkers in the era of personalized medicine. Fam Cancer. 2016 Feb 13 (Epub ahead of print).
http://dx.doi.org/10.1007/s10689-016-9884-6.
Fransen K, Klintenas M, Osterstrom A, Dimberg J, Monstein HJ, Soderkvist P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25(4):527-33. http://dx.doi.org/10.1093/carcin/bgh049.
Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48(10):1466-75. http://dx.doi.org/10.1016/j.ejca.2012.02.057.
French AJ, Sargent DJ, Burgart LJ, Foster NR, Kabat BF, Goldberg R, et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res. 2008;14(11):3408-15.
http://dx.doi.org/10.1158/1078-0432.CCR-07-1489.
Ogino S, Shima K, Meyerhardt JA, McCleary NJ, Ng K, Hollis D, et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res. 2012;18(3):890-900.
http://dx.doi.org/10.1158/1078-0432.CCR-11-2246.
Yokota T, Ura T, Shibata N, Takahari D, Shitara K, Nomura M, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104(5):856-62. http://dx.doi.org/10.1038/bjc.2011.19.
Vogelaar F, Van Erning F, Reimers M, Van Der Linden J, Pruijt J, Van Den Brule A, et al. The prognostic value of Microsatellite Instability, KRAS, BRAF and PIK3CA mutations in stage II colon cancer patients. Mol Med. 2015:1-26. DOI: 10.2119/molmed.2015.00220.
Lin CC, Lai YL, Lin TC, Chen WS, Jiang JK, Yang SH, et al. Clinicopathologic features and prognostic analysis of MSI-high colon cancer. Int J Colorectal Dis. 2012;27(3):277-86.
http://dx.doi.org/10.1007/s00384-011-1341-2.
Kaur G, Masoud A, Raihan N, Radzi M, Khamizar W, Kam LS. Mismatch repair genes expression defects & association with clinicopathological characteristics in colorectal carcinoma. Indian J Med Res. 2011;134:186-92.
Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158(2):527-35. http://dx.doi.org/10.1016/S0002-9440(10)63994-6.
Lin CC, Lin JK, Lin TC, Chen WS, Yang SH, Wang HS, et al. The prognostic role of microsatellite instability, codon-specific KRAS, and BRAF mutations in colon cancer. J Surg Oncol. 2014;110(4):451-7. http://dx.doi.org/10.1002/jso.23675.
Iachetta F, Domati F, Reggiani-Bonetti L, Barresi V, Magnani G, Marcheselli L, et al. Prognostic relevance of microsatellite instability in pT3N0M0 colon cancer: a population-based study. Intern Emerg Med. 2016;11(1):41-6. DOI: 10.1007/s11739-015-1285-6.
Deschoolmeester V, Van Damme N, Baay M, Claes K, Van Marck E, Baert FJ, et al. Microsatellite instability in sporadic colon carcinomas has no independent prognostic value in a Belgian study population. Eur J Cancer. 2008;44(15):2288-95.
http://dx.doi.org/10.1016/j.ejca.2008.06.043.
Shin US, Cho SS, Moon SM, Park SH, Jee SH, Jung EJ, et al. Is microsatellite instability really a good prognostic factor of colorectal cancer? Ann Coloproctol. 2014;30(1):28-34.
http://dx.doi.org/10.3393/ac.2014.30.1.28.
Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219-26.
http://dx.doi.org/10.1200/JCO.2009.27.1825.
Saridaki Z, Souglakos J, Georgoulias V. Prognostic and predictive significance of MSI in stages II/III colon cancer. World J Gastroenterol. 2014;20(22):6809-14.
http://dx.doi.org/10.3748/wjg.v20.i22.6809.
Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103(11):863-75.
http://dx.doi.org/10.1093/jnci/djr153.
Samowitz WS, Curtin K, Wolff RK, Tripp SR, Caan BJ, Slattery ML. Microsatellite instability and survival in rectal cancer. Cancer Causes Control. 2009;20(9):1763-8.
http://dx.doi.org/10.1007/s10552-009-9410-3.
Farina-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VE, Rutten HJ, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol. 2010;21(12):2396-402.
http://dx.doi.org/10.1093/annonc/mdq258.
Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28(3):466-74.
http://dx.doi.org/10.1200/JCO.2009.23.3452.
Taieb J, Zaanan A, Le Malicot K, Julie C, Blons H, Mineur L, et al. Prognostic effect of BRAF and KRAS mutations in patients with Stage III colon cancer treated with Leucovorin, Fluorouracil, and Oxaliplatin with or without Cetuximab: a post hoc analysis of the PETACC-8 Trial. JAMA Oncol. 2016;14:1-11.
Downloads
Additional Files
Published
How to Cite
Accepted 2016-04-23
Published 2016-11-10









