Combination effect of cytochrome P450 1A1 gene polymorphisms on uterine leiomyoma: A case-control study
DOI:
https://doi.org/10.17305/bjbms.2016.1245Keywords:
Cytochrome P450 1A1, haplotype, gene, polymorphism, uterine leiomyomaAbstract
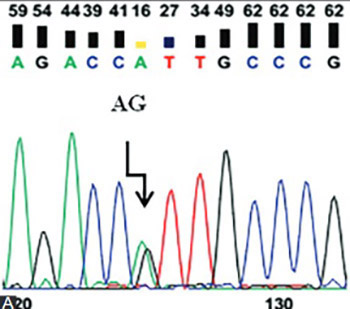
Uterine leiomyoma (UL) is an estrogen-dependent neoplasm of the uterus, and estrogen metabolizing enzymes affect its progression. This study aimed to evaluate the association between two single-nucleotide polymorphisms of cytochrome P450 1A1 (CYP1A1) gene and UL risk. The study consisted of 105 patients with UL and 112 healthy women as controls. Ile462Val (A/G) and Asp449Asp (T/C) polymorphisms of CYP1A1 gene were analyzed by DNA sequencing and polymerase chain reaction-restriction fragment length polymorphism methods, respectively. The findings indicated no association between Ile462Val (A/G) and Asp449Asp (T/C) polymorphisms of CYP1A1 gene and UL (p < 0.05). However, the combination effect of TT/AG genotypes of the Asp449Asp (T/C) and Ile462Val (A/G) polymorphisms was associated with 4.3-fold higher risk of UL. In addition, haplotype analysis revealed that TG haplotype of the Asp449Asp (T/C) and Ile462Val (A/G) polymorphisms could increase the UL risk nearly 4.9-fold. Asp449Asp (T/C) and Ile462Val (A/G) polymorphisms of CYP1A1 gene were not associated with UL susceptibility; however, the combination of the TT/AG genotypes and TG haplotype could increase the UL risk.
Citations
Downloads
References
. Salimi S, Khodamian M, Narooie-Nejad M, Hajizadeh A, Fazeli K, Namazi L, et al. Association of polymorphisms and haplotypes in the cytochrome P450 1B1 gene with uterine leiomyoma: A case control study. Biomed Rep 2015;3(2):201-6.
. Wang F, Chen J, Wang L, Ma Y, Mayinuer N. CYP1A1 genetic polymorphisms and uterine leiomyoma risk: A meta-analysis. Int J Clin Exp Med 2015;8(3):3590-4.
. Feng Y, Lin X, Zhou S, Xu N, Yi T, Zhao X. The associations between the polymorphisms of the ER-a gene and the risk of uterine leiomyoma (ULM). Tumour Biol 2013;34(5):3077-82. http://dx.doi.org/10.1007/s13277-013-0874-0.
. Mäkinen N, Vahteristo P, Kämpjärvi K, Arola J, Bützow R, Aaltonen LA. MED12 exon 2 mutations in histopathological uterine leiomyoma variants. Eur J Hum Genet 2013;21(11):1300-3. http://dx.doi.org/10.1038/ejhg.2013.33.
. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007;87(4):725-36. http://dx.doi.org/10.1016/j.fertnstert.2007.01.093.
. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997;138(3):863-70. http://dx.doi.org/10.1210/en.138.3.863, http://dx.doi.org/10.1210/endo.138.3.4979.
. Wang H, Wu X, Englund K, Masironi B, Eriksson H, Sahlin L. Different expression of estrogen receptors alpha and beta in human myometrium and leiomyoma during the proliferative phase of the menstrual cycle and after GnRHa treatment. Gynecol Endocrinol 2001;15(6):443-52. http://dx.doi.org/10.1080/713602983, http://dx.doi.org/10.1080/gye.15.6.443.452.
. Somner J, McLellan S, Cheung J, Mak YT, Frost ML, Knapp KM, et al. Polymorphisms in the P450 c17 (17-hydroxylase/17,20-Lyase) and P450 c19 (aromatase) genes: Association with serum sex steroid concentrations and bone mineral density in postmenopausal women. J Clin Endocrinol Metab 2004;89(1):344-51. http://dx.doi.org/10.1210/jc.2003-030164.
. Stiborová M, Martínek V, Rýdlová H, Koblas T, Hodek P. Expression of cytochrome P450 1A1 and its contribution to oxidation of a potential human carcinogen 1-phenylazo-2-naphthol (Sudan I) in human livers. Cancer Lett 2005;220(2):145-54. http://dx.doi.org/10.1016/j.canlet.2004.07.036.
. Ashton KA, Proietto A, Otton G, Symonds I, McEvoy M, Attia J, et al. Polymorphisms in genes of the steroid hormone biosynthesis and metabolism pathways and endometrial cancer risk. Cancer Epidemiol 2010;34(3):328-37. http://dx.doi.org/10.1016/j.canep.2010.03.005.
. Sowers MR, Wilson AL, Kardia SR, Chu J, McConnell DS. CYP1A1 and CYP1B1 polymorphisms and their association with estradiol and estrogen metabolites in women who are premenopausal and perimenopausal. Am J Med 2006;119(9 Suppl 1):S44-51. http://dx.doi.org/10.1016/j.amjmed.2006.07.006.
. Khvostova EP, Pustylnyak VO, Gulyaeva LF. Genetic polymorphism of estrogen metabolizing enzymes in Siberian women with breast cancer. Genet Test Mol Biomarkers 2012;16(3):167-73. http://dx.doi.org/10.1089/gtmb.2011.0131.
. Michnovicz JJ, Rosenberg DW. Oxidative metabolism of estrogens in rat intestinal mitochondria. Biochem Pharmacol 1992;43(8):1847-52. http://dx.doi.org/10.1016/0006-2952(92)90720-4.
. Arvanitis DA, Koumantakis GE, Goumenou AG, Matalliotakis IM, Koumantakis EE, Spandidos DA. CYP1A1, CYP19, and GSTM1 polymorphisms increase the risk of endometriosis. Fertil Steril 2003;79(Suppl 1):702-9.
http://dx.doi.org/10.1016/S0015-0282(02)04817-3.
. Aktas D, Guney I, Alikasifoglu M, Yüce K, Tuncbilek E, Ayhan A. CYP1A1 gene polymorphism and risk of epithelial ovarian neoplasm. Gynecol Oncol 2002;86(2):124-8. http://dx.doi.org/10.1006/gyno.2002.6720.
. Napoli N, Villareal DT, Mumm S, Halstead L, Sheikh S, Cagaanan M, et al. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res 2005;20(2):232-9. http://dx.doi.org/10.1359/JBMR.041110.
. Herr D, Bettendorf H, Denschlag D, Keck C, Pietrowski D. Cytochrome P2A13 and P1A1 gene polymorphisms are associated with the occurrence of uterine leiomyoma. Arch Gynecol Obstet 2006;274(6):367-71.
http://dx.doi.org/10.1007/s00404-006-0201-8.
. Ye Y, Cheng X, Luo H-B, Liu L, Li Y-B, Hou Y-P. CYP1A1 and CYP1B1 genetic polymorphisms and uterine leiomyoma risk in Chinese women. J Assist Reprod Genet 2008;25(8):389-94. http://dx.doi.org/10.1007/s10815-008-9246-x.
. Barão MA, Oliveira E, Gomes MT, da Silva ID, Sartori MG, Girão MJ, et al. The role of MSP I CYP1A1 gene polymorphism in the development of uterine fibroids. Fertil Steril 2010;94(7):2783-5. http://dx.doi.org/10.1016/j.fertnstert.2010.05.034.
. El-Shennawy GA, Elbialy AA, Isamil AE, El Behery MM. Is genetic polymorphism of ER-a, CYP1A1, and CYP1B1 a risk factor for uterine leiomyoma? Arch Gynecol Obstet 2011;283(6):1313-8. http://dx.doi.org/10.1007/s00404-010-1550-x.
. Zhou C, Lin L, Zhang Y, Xu T, Zhang L. A relevance study on uterine leiomyoma and gene polymorphisms of CYP1A1 MspI and SULT1A1 Arg213His. Chin J Clin Obstet Gynecol 2011;4:287-90. DOI: 10.3969/j.issn.1672-1861.2011.04.014.
. Shen Y, Ren ML, Xu J, Xu Q, Ding YQ, Wu ZC, et al. A multicenter case-control study on screening of single nucleotide polymorphisms in estrogen-metabolizing enzymes and susceptibility to uterine leiomyoma in Han Chinese. Gynecol Obstet Invest 2014;77(4):224-30. http://dx.doi.org/10.1159/000360083.
. Shen Y, Xu Q, Ren M, Cai Y, Xu J. Role of single nucleotide polymorphisms in estrogen-metabolizing enzymes and susceptibility to uterine leiomyoma in Han Chinese: A case-control study. J Obstet Gynaecol Res 2014;40(4):1077-84. http://dx.doi.org/10.1111/jog.12275.
. Pritchard JK, Przeworski M. Linkage disequilibrium in humans: Models and data. Am J Hum Genet 2001;69(1):1-14. http://dx.doi.org/10.1086/321275.
. Gaunt TR, Rodríguez S, Day IN. Cubic exact solutions for the estimation of pairwise haplotype frequencies: Implications for linkage disequilibrium analyses and a web tool 'CubeX'. BMC Bioinformatics 2007;8:428.
http://dx.doi.org/10.1186/1471-2105-8-428.
. Taghizade Mortezaee F, Tabatabaiefar MA, Hashemzadeh Chaleshtori M, Miraj S. Lack of association between ESR1 and CYP1A1 gene polymorphisms and susceptibility to uterine leiomyoma in female patients of Iranian descent. Cell J 2014;16(2):225-30.
Downloads
Additional Files
Published
How to Cite
Accepted 2016-05-04
Published 2016-08-02









