Decitabine suppresses tumor growth by activating mouse mammary tumor virus and interferon-β pathways
DOI:
https://doi.org/10.17305/bb.2025.12852Keywords:
Decitabine , DNA methyltransferase inhibitor, mouse mammary tumor virus, interferon, tumor, cancer, 4T1, MC38, interferon regulatory factor 7Abstract
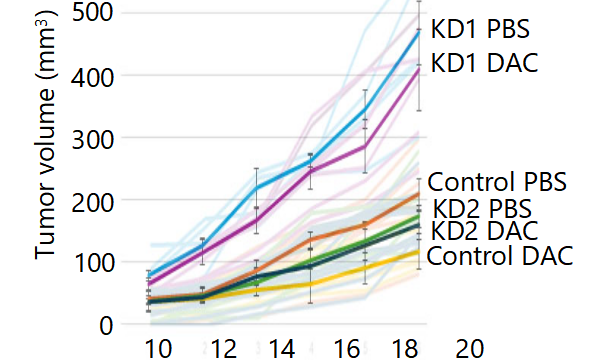
Decitabine (DAC), a DNA methyltransferase inhibitor (DNMTi), is clinically effective in hematological malignancies such as myelodysplastic syndrome and acute myeloid leukemia, but its precise antineoplastic mechanisms remain incompletely understood. Beyond promoter demethylation, DAC is known to activate endogenous retroviruses (ERVs) and trigger type I interferon (IFN-I) responses, a phenomenon known as viral mimicry. The aim of this study was to investigate the roles of the mouse mammary tumor virus (MMTV) and interferon-β (IFN-β) in DAC-mediated tumor suppression. We employed two murine tumor models—4T1 mammary carcinoma and MC38 colon adenocarcinoma—in syngeneic immunocompetent mice, immunodeficient nude mice, and in vitro cultures. RNA and protein expression were assessed by quantitative PCR and immunoblotting, while functional contributions of MMTV and IFN-β were tested using short hairpin RNA (shRNA) knockdowns. DAC treatment suppressed tumor growth and pulmonary metastasis in vivo and inhibited cancer cell proliferation in vitro. It induced transcription of MMTV and expression of IFN-β, with a strong negative correlation between MMTV Env protein levels and tumor mass. Knockdown of either MMTV or IFN-β conferred resistance to DAC, confirming their functional roles. Reciprocal regulation was observed: MMTV knockdown reduced IFN-β expression, while IFN-β knockdown increased MMTV Env accumulation. Furthermore, DAC upregulated interferon regulatory factor 7 (IRF7), but this effect declined during prolonged treatment, suggesting a temporally restricted therapeutic window. In conclusion, our findings provide in vivo support for the viral mimicry hypothesis and demonstrate that MMTV and IFN-β contribute to DAC-mediated tumor suppression. The observed IRF7 downregulation and potential induction of immune checkpoints highlight the importance of therapeutic strategies combining DNMTis with immune checkpoint blockade to sustain antineoplastic efficacy.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Ryan Johnson, Andrew Brola, Cade Wycoff, William Wycoff, Seth Neumeyer, Richard Tuttle, Sarah Light, Jiayi Li, Stephen Christensen, Yingguang Liu

This work is licensed under a Creative Commons Attribution 4.0 International License.









