Lactylation in ischemic brain injury—metabolic mechanisms, neuroinflammation, and therapeutic targets: A review
DOI:
https://doi.org/10.17305/bb.2025.12955Keywords:
Lactylation modification, cerebral ischemic injury, epigenetic modification, hypoxia-reperfusion, treatment strategiesAbstract
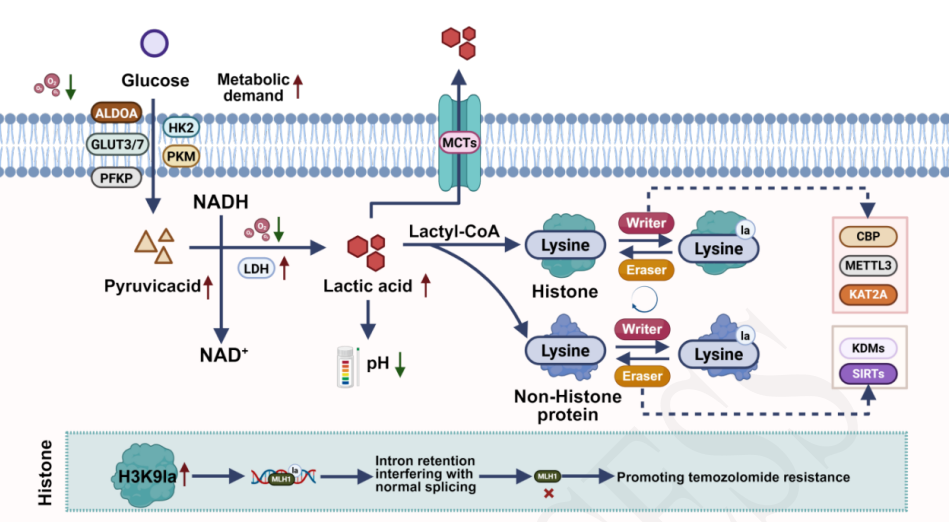
Cerebral ischemic injury, a major cause of mortality and disability, results from reduced or interrupted blood flow to the brain, most commonly in ischemic stroke. Insufficient oxygen and nutrient supply disrupts cellular metabolism, leading to neuronal death, neurological dysfunction, and lasting impairments. Current therapeutic strategies, including thrombolysis, mechanical thrombectomy, and anticoagulation, primarily aim to restore perfusion and provide neuroprotection by preserving the ischemic penumbra. While these interventions can partially rescue viable tissue in the acute phase, their effectiveness is constrained by narrow therapeutic windows, low recanalization rates, and contraindications, leaving significant unmet clinical needs. Consequently, the search for novel, targeted approaches has become a central focus of ischemic stroke research. Recent discoveries have identified lactylation, a newly recognized post-translational modification derived from lactate, as a key regulator of gene expression, protein function, and metabolic reprogramming. Once regarded as a simple glycolytic byproduct, lactate is now known to act as both an alternative energy substrate and a signaling molecule, influencing neuronal metabolism, antioxidant defense, and inflammatory responses. In ischemic brain injury, lactylation modifications of histone and non-histone proteins may either protect neurons—by supporting energy homeostasis, regulating stress-responsive genes, and suppressing apoptosis—or exacerbate injury through neuroinflammation, excitotoxicity, and immune evasion. Evidence indicates that the outcomes of lactylation depend on lactate concentration, timing of accumulation, cell type, and the balance between “writer” and “eraser” enzymes. Therefore, lactylation emerges as a promising yet complex therapeutic target in cerebral ischemia. Modulating lactate metabolism and its downstream modifications offers new opportunities to expand the therapeutic window, attenuate neuronal injury, and improve recovery. This review summarizes the molecular mechanisms linking lactate and lactylation to ischemic injury, highlights current contradictions in experimental findings, and explores the potential of targeting lactylation pathways for innovative treatment strategies.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Xinchen Ji, Jing Lu, Ke Wang, Yan Guo, Dexi Zhao, Miao Liu

This work is licensed under a Creative Commons Attribution 4.0 International License.









