Mitochondrial dysfunction triggers Zbp1-mediated necroptosis and inflammation in acute lung injury
DOI:
https://doi.org/10.17305/bb.2025.13046Keywords:
Acute lung injury, alveolar macrophages, mitochondria, Zbp1Abstract
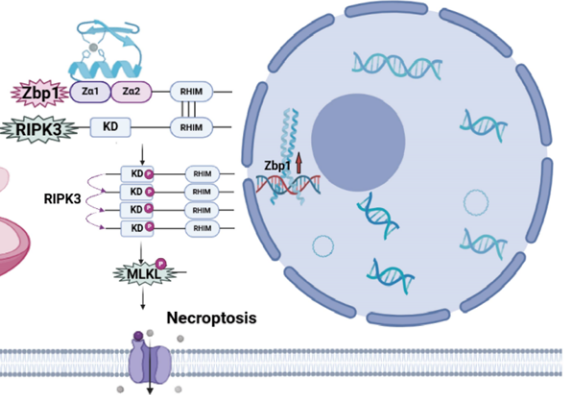
Acute lung injury (ALI) is driven by dysregulated inflammation, but how mitochondrial damage engages necroptosis in alveolar macrophages remains unclear. We aimed to define the mechanistic link between mitochondrial impairment and Zinc finger protein 1 (Zbp1)–mediated necroptosis in the murine alveolar macrophage–like cell line (MH-S). MH-S cells were stimulated with lipopolysaccharide (LPS) and profiled by RNA sequencing; necroptotic death was quantified by Calcein-AM/propidium iodide (PI) staining and lactate dehydrogenase (LDH) release, Zbp1 localization was examined by immunofluorescence microscopy, and Zbp1, receptor-interacting protein kinase 3 (RIPK3)/phospho-RIPK3 (p-RIPK3) and mixed lineage kinase domain-like protein (MLKL)/phospho-MLKL (p-MLKL) were measured by Western blotting. Mitochondrial status was assessed by mitochondrial reactive oxygen species (mtROS), mitochondrial membrane potential (ΔΨm; JC-1), mitochondrial permeability transition pore (MPTP) opening, adenosine triphosphate (ATP) content, and the markers ATP synthase F1 subunit alpha (ATP5a1), mitochondrial transcription factor A (TFAM), and translocase of outer mitochondrial membrane 20 (TOMM20); inflammatory responses were quantified by flow cytometry and qPCR. The mitochondria-targeted antioxidant Mito-TEMPO was used to interrogate the role of oxidative stress. LPS markedly increased Zbp1 transcription, coincident with upregulation of pro-inflammatory genes and activation of necroptosis; mitochondrial damage and elevated mtROS were critical upstream events for Zbp1 induction, driving RIPK3 and MLKL phosphorylation, necroptosis, and cytokine release. Mito-TEMPO restored mitochondrial function, lowered mtROS, downregulated Zbp1 and its necroptotic effectors (p-RIPK3, p-MLKL), and significantly reduced both necroptotic injury and inflammatory output. Collectively, mitochondrial dysfunction–driven mtROS initiates the Zbp1/RIPK3/MLKL necroptotic axis in alveolar macrophages, thereby amplifying pulmonary inflammation in ALI; targeting mtROS may mitigate necroptosis and protect against lung injury.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2025 Mi Zhou, Yuehan Li, Yinying Ren, Yan Li, JinYing Xiang, Fang Deng, Gang Geng, Jian Luo, Jinyue Yu, Zhou Fu, Fengxia Ding, Bo Liu

This work is licensed under a Creative Commons Attribution 4.0 International License.









