Mitochondrial dysfunction, reactive oxygen species, and diabetes mellitus – A triangular relationship: A review
DOI:
https://doi.org/10.17305/bb.2025.13145Keywords:
Oxidative stress, reactive oxygen species, mitochondrial dysfunction, diabetes mellitus, cardiovascular complicationsAbstract
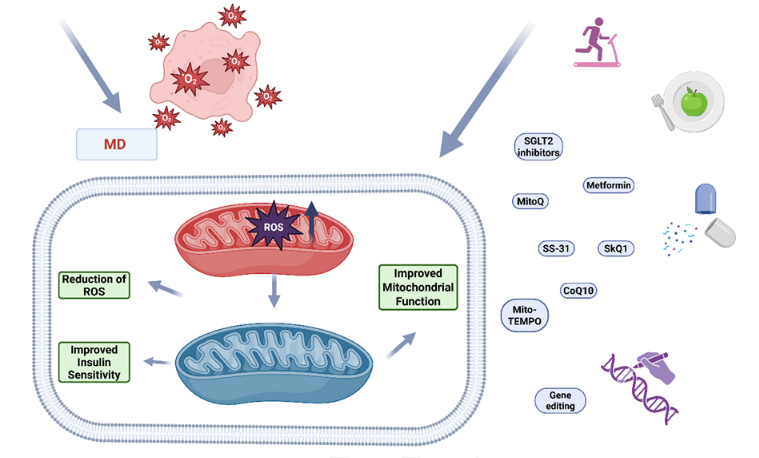
Diabetes mellitus (DM) disrupts cellular homeostasis and is characterized by mitochondrial structural and functional impairments similar to those found in other metabolic disorders. Mitochondrial dysfunction (MD) leads to the excessive production of reactive oxygen species (ROS), which are central to the progression of cardiovascular (CV) disease—the leading cause of mortality associated with DM. ROS-driven oxidative stress (OS) is implicated in cardiac injury in both clinical and experimental contexts. This review synthesizes recent literature on the role of MD in the development and progression of DM and its associated CV complications, highlighting disrupted pathways that regulate the balance between ROS production and antioxidant defenses. We summarize alterations in mitochondrial dynamics—including fusion, fission, and mitophagy—mtDNA damage, and impaired oxidative phosphorylation characterized by dysregulated mitochondrial membrane potential (ΔΨm), electron transport chain (ETC) defects, uncoupling, and substrate overload. Additionally, we discuss hyperglycemia-activated pathways such as polyol flux, AGE–RAGE interactions, protein kinase C/nicotinamide adenine dinucleotide phosphate (PKC/NADPH) oxidase activation, and poly(ADP-ribose) polymerase 1 (PARP-1)-mediated glyceraldehyde-3-phosphate dehydrogenase (GAPDH) inhibition, which contribute to inflammation, endothelial dysfunction, β-cell failure, insulin resistance, and micro/macrovascular injury. Diagnostic and biomarker strategies encompass mtDNA analysis, bioenergetic assays, metabolomics, proteomics, and imaging techniques including PET, MRI, and NIRS. Therapeutic approaches aimed at restoring mitochondrial function and mitigating OS include mitochondria-targeted antioxidants (such as MitoQ, CoQ10, SkQ1, SS-31, and Mito-TEMPO), metabolic drugs (including metformin and SGLT2 inhibitors), lifestyle modifications, and emerging gene-editing technologies. The interplay between mitochondria, ROS, and DM reflects a tightly regulated aspect of cellular physiology; while targeted and personalized strategies hold promise, they necessitate rigorous evaluation.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Mia Manojlovic, Sonja Zafirovic, Dragana Tomic Naglic, Edita Stokic, Manfredi Rizzo, Jasjit S. Suri, Esma Isenovic

This work is licensed under a Creative Commons Attribution 4.0 International License.









