Nitric oxide synthesis-promoting effects of valsartan in human umbilical vein endothelial cells via the Akt/adenosine monophosphate-activated protein kinase/endothelial nitric oxide synthase pathway
DOI:
https://doi.org/10.17305/bjbms.2017.1319Keywords:
Valsartan, nitric oxide, protein kinase B, adenosine monophosphate-activated protein kinase, endothelial nitric oxide synthaseAbstract
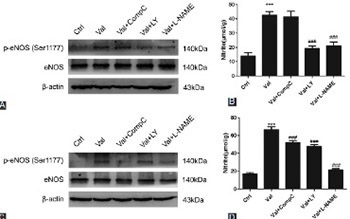
Valsartan (VAL), an antagonist of angiotensin II receptor type 1, has antihypertensive and multiple cardiovascular protective effects. The pleiotropic functions of VAL are related to the increased synthesis and biological activity of intravascular nitric oxide (NO). In this study, the role and mechanisms of VAL in the synthesis of NO were examined in human umbilical vein endothelial cells (HUVECs). Ten µmol/L of VAL was used to treat EA.hy926 cells for 30 minutes, 1, 3, 6, 12, and 24 hours, and three concentrations of VAL (i.e., 10, 1, and 0.1 µmol/L) were used to treat EA.hy926 cells for 24 hours. The cells were divided into five groups: control, VAL, VAL + Compound C (adenosine monophosphate-activated protein kinase [AMPK] inhibitor, 1 µmol/L), VAL + LY294002 (Akt [protein kinase B] inhibitor, 10 µmol/L), and VAL + L-nitro-arginine methyl ester (L-NAME, endothelial NO synthase [eNOS] inhibitor, 500 µmol/L) groups. The NO content in the VAL-treated HUVEC line (EA.hy926) was detected using the nitrate reductase method, and western blot was used to detect the phosphorylation of Akt, AMPK, and eNOS, as well as the changes in total protein levels. VAL increased NO synthesis in EA.hy926 cells in time- and dose-dependent manners (p < 0.05) and the intracellular phosphorylation levels of Akt, AMPK, and eNOS at the corresponding time points. LY294002, Compound C, and L-NAME could inhibit the VAL-promoted NO synthesis. VAL activated Akt, AMPK, and eNOS, thus promoting NO synthesis and playing a protective role in endothelial cells. These results partially explained the mechanisms underlying the cardiovascular protective effects of VAL.
Citations
Downloads
References
Sander GE, Giles TD. Nebivolol and valsartan as a fixed-dose combination for the treatment of hypertension. Expert Opin Pharmacother 2015;16(5):763-70. http://dx.doi.org/10.1517/14656566.2015.1020790.
Xu Y, Hu X, Wang L, Jiang Z, Liu X, Yu H, et al. Preconditioning via angiotensin Type 2 receptor activation improves therapeutic efficacy of bone marrow mononuclear cells for cardiac repair. PLoS One 2013;8(12):e82997. http://dx.doi.org/10.1371/journal.pone.0082997.
Kalinowski L, Matys T, Chabielska E, Buczko W, Malinski T. Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertension 2002;40(4):521-7. http://dx.doi.org/10.1161/01.HYP.0000034745.98129.EC.
Duan L, Lei H, Zhang Y, Wan B, Chang J, Feng Q, et al. Calcitonin gene-related peptide improves hypoxia-induced inflammation and apoptosis via nitric oxide in H9c2 cardiomyoblast cells. Cardiology 2016;133(1):44-53. http://dx.doi.org/10.1159/000439123.
Sunshine SB, Dallabrida SM, Durand E, Ismail NS, Bazinet L, Birsner AE, et al. Endostatin lowers blood pressure via nitric oxide and prevents hypertension associated with VEGF inhibition. Proc Natl Acad Sci U S A 2012;109(28):11306-11. http://dx.doi.org/10.1073/pnas.1203275109.
Campelo AE, Cutini PH, Massheimer VL. Testosterone modulates platelet aggregation and endothelial cell growth through nitric oxide pathway. J Endocrinol 2012;213(1):77-87. http://dx.doi.org/10.1530/JOE-11-0441.
Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 2015;14(9):623-41. http://dx.doi.org/10.1038/nrd4623.
Su KH, Tsai JY, Kou YR, Chiang AN, Hsiao SH, Wu YL, et al. Valsartan regulates the interaction of angiotensin II Type 1 receptor and endothelial nitric oxide synthase via Src/PI3K/Aktsignalling. Cardiovasc Res 2009;82(3):468-75. http://dx.doi.org/10.1093/cvr/cvp091.
Tsai HY, Lin CP, Huang PH, Li SY, Chen JS, Lin FY, et al. Coenzyme Q10 attenuates high glucose-induced endothelial progenitor cell dysfunction through AMP-activated protein kinase pathways. J Diabetes Res 2016;2016:6384759. http://dx.doi.org/10.1155/2016/6384759.
Lee CH, Lee SD, Ou HC, Lai SC, Cheng YJ. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway. Int J Mol Sci 2014;15(6):10334-49. http://dx.doi.org/10.3390/ijms150610334.
Ha YM, Park EJ, Kang YJ, Park SW, Kim HJ, Chang KC. Valsartan independent of AT1 receptor inhibits tissue factor, TLR-2 and -4 expression by regulation of Egr-1 through activation of AMPK in diabetic conditions. J Cell Mol Med 2014;18(10):2031-43. http://dx.doi.org/10.1111/jcmm.12354.
Zhong X, Xiu LL, Wei GH, Liu YY, Su L, Cao XP, et al. Bezafibrate enhances proliferation and differentiation of osteoblastic MC3T3-E1 cells via AMPK and eNOS activation. Acta Pharmacol Sin 2011;32(5):591-600. http://dx.doi.org/10.1038/aps.2011.15.
Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev 2010;9(12):830-4. http://dx.doi.org/10.1016/j.autrev.2010.07.016.
Taddei S, Virdis A, Mattei P, Salvetti A. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension 1993;21(6 Pt 2):929-33. http://dx.doi.org/10.1161/01.HYP.21.6.929.
Wyatt AW, Steinert JR, Mann GE. Modulation of the L-arginine/nitric oxide signalling pathway in vascular endothelial cells. Biochem Soc Symp 2004;71:143-56. http://dx.doi.org/10.1042/bss0710143.
Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortin D, et al. Activation of AMP kinase alpha 1 subunit induces aortic vasorelaxation in mice. J Physiol 2007;581(Pt 3):1163-71. http://dx.doi.org/10.1113/jphysiol.2007.132589.
Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 2003;278(33):31000-6. http://dx.doi.org/10.1074/jbc.M300643200.
Ewart MA, Kennedy S. AMPK and vasculoprotection. Pharmacol Ther 2011;131(2):242-53. http://dx.doi.org/10.1016/j.pharmthera.2010.11.002.
Labuzek K, Liber S, Gabryel B, Buldak L, Okopien B. Ambivalent effects of compound C (dorsomorphin) on inflammatory response in LPS-stimulated rat primary microglial cultures. Naunyn Schmiedebergs Arch Pharmacol 2010;381(1):41-57. http://dx.doi.org/10.1007/s00210-009-0472-2.
Yehuda I, Madar Z, Leikin-Frenkel A, Tamir S. Glabridin, an isoflavan from licorice root, downregulates iNOS expression and activity under high-glucose stress and inflammation. Mol Nutr Food Res 2015;59(6):1041-52. http://dx.doi.org/10.1002/mnfr.201400876.
Cortese-Krott MM, Kulakov L, Opländer C, Kolb-Bachofen V, Kröncke KD, Suschek CV. Zinc regulates iNOS-derived nitric oxide formation in endothelial cells. Redox Biol 2014;2:945-54. http://dx.doi.org/10.1016/j.redox.2014.06.011.
Thibert C, Perret C, Billaud M. AMPK potentiation by LKB1 isoforms. Oncotarget 2015;6(34):35139-40. DOI: 10.18632/oncotarget.6127.
Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 2005;280(32):29060-6. http://dx.doi.org/10.1074/jbc.M503824200.
Herrero-Martín G, Høyer-Hansen M, García-García C, Fumarola C, Farkas T, López-Rivas A, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J 2009;28(6):677-85. http://dx.doi.org/10.1038/emboj.2009.8.
Iwashita M, Sakoda H, Kushiyama A, Fujishiro M, Ohno H, Nakatsu Y, et al. Valsartan, independently of AT1 receptor or PPAR gamma, suppresses LPS-induced macrophage activation and improves insulin resistance in cocultured adipocytes. Am J Physiol Endocrinol Metab 2012;302(3):E286-96. http://dx.doi.org/10.1152/ajpendo.00324.2011.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-10-28
Published 2017-05-20









