Annexins and autoantibodies in autoimmune diseases – Insights into SLE, APS and RA: A review
DOI:
https://doi.org/10.17305/bb.2026.13546Keywords:
Annexin family, autoantibodies, autoimmune diseases, systemic lupus erythematosus, antiphospholipid syndromeAbstract
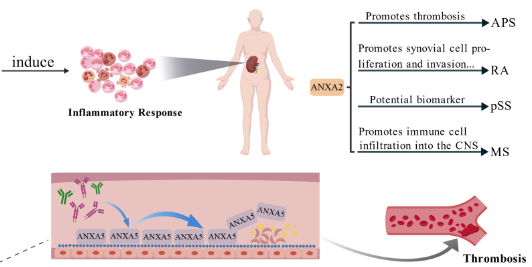
Autoimmune diseases are becoming increasingly prevalent and can cause multi-organ damage through dysregulated immune responses to self-antigens. This review aims to summarize the roles of annexin family proteins and annexin autoantibodies in the mechanisms of autoimmune diseases, as well as their potential diagnostic and therapeutic applications. A targeted PubMed search conducted on August 31, 2025, utilized annexin- and disease-related terms without year restrictions, focusing on English-language, peer-reviewed studies involving humans or recognized animal models. Evidence suggests that Annexin A1 (ANXA1) and formyl peptide receptor 2 (FPR2) signaling can influence inflammatory and T-cell responses. Additionally, Annexin A2 (ANXA2) is associated with organ-targeted injury, such as lupus nephritis (LN) in systemic lupus erythematosus (SLE), through its interactions with anti-double-stranded DNA antibodies (anti-dsDNA). Annexin A5 (ANXA5) serves as an anticoagulant phospholipid "shield," which can be compromised by antiphospholipid antibodies (aPLs), contributing to thrombosis and obstetric complications in antiphospholipid syndrome (APS) and increasing vascular risk in SLE. In rheumatoid arthritis (RA), ANXA1 exhibits context-dependent effects, while ANXA2 promotes synovial proliferation, invasion, and angiogenesis. Dysregulation of annexins has also been observed in primary Sjögren's syndrome (pSS), multiple sclerosis (MS), and systemic sclerosis (SSc). Additionally, the emerging utility of anti-ANXA1, anti-ANXA2, and anti-ANXA5 autoantibodies for phenotyping and risk stratification, including in seronegative antiphospholipid syndrome (SNAPS), highlights their clinical relevance. Overall, annexins and their autoantibodies represent promising biomarkers and therapeutic targets; however, the heterogeneity of assays and the limited availability of prospective multicenter data currently hinder clinical translation.
Citations
Downloads
References
Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878–90. https://doi.org/10.1016/S0140-6736(23)00457-9
Luo B, Xiang D, Ji X, Chen X, Li R, Zhang S, et al. The anti-inflammatory effects of exercise on autoimmune diseases: A 20-year systematic review. J Sport Health Sci. 2024;13(3):353–67.
https://doi.org/10.1016/j.jshs.2024.02.002
Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol. 2023;80:102266. https://doi.org/10.1016/j.coi.2022.102266
Gerke V, Gavins FNE, Geisow M, Grewal T, Jaiswal JK, Nylandsted J, et al. Annexins–a family of proteins with distinctive tastes for cell signaling and membrane dynamics. Nat Commun. 2024;15(1):1574. https://doi.org/10.1038/s41467-024-45954-0
Qian Z, Li Z, Peng X, Mao Y, Mao X, Li J. Annexin A: Cell death, inflammation, and translational medicine. J Inflamm Res. 2025;18:5655–72. https://doi.org/10.2147/JIR.S511439
Huang Y, Jia M, Yang X, Han H, Hou G, Bi L, et al. Annexin A2: The diversity of pathological effects in tumorigenesis and immune response. Int J Cancer. 2022;151(4):497–509.
https://doi.org/10.1002/ijc.34048
Zhang H, Zhang Z, Guo T, Chen G, Liu G, Song Q, et al. Annexin A protein family: focusing on the occurrence, progression and treatment of cancer. Front Cell Dev Biol. 2023;11:1141331.
https://doi.org/10.3389/fcell.2023.1141331
Hu J, Chen L, Ruan J, Chen X. The role of the annexin A protein family at the maternal-fetal interface. Front Endocrinol (Lausanne). 2024;15:1314214. https://doi.org/10.3389/fendo.2024.1314214
Fang L, Liu C, Jiang ZZ, Wang M, Geng K, Xu Y, et al. Annexin A1 binds PDZ and LIM domain 7 to inhibit adipogenesis and prevent obesity. Signal Transduct Target Ther. 2024;9(1):218.
https://doi.org/10.1038/s41392-024-01930-0
Pan H, Guo Z, Lv P, Hu K, Wu T, Lin Z, et al. Proline/serine-rich coiled-coil protein 1 inhibits macrophage inflammation and delays atherosclerotic progression by binding to Annexin A2. Clin Transl Med. 2023;13(3):e1220. https://doi.org/10.1002/ctm2.1220
Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82(2):331–71.
https://doi.org/10.1152/physrev.00030.2001
Mirsaeidi M, Gidfar S, Vu A, Schraufnagel D. Annexins family: insights into their functions and potential role in pathogenesis of sarcoidosis. J Transl Med. 2016;14:89.
https://doi.org/10.1186/s12967-016-0843-7
Grewal T, Rentero C, Enrich C, Wahba M, Raabe CA, Rescher U. Annexin animal models–from fundamental principles to translational research. Int J Mol Sci. 2021;22(7):3439.
https://doi.org/10.3390/ijms22073439
Araújo TG, Mota STS, Ferreira HSV, Ribeiro MA, Goulart LR, Vecchi L. Annexin A1 as a regulator of immune response in cancer. Cells. 2021;10(9):2245. https://doi.org/10.3390/cells10092245
Sousa SO, Santos MRD, Teixeira SC, Ferro EAV, Oliani SM. Annexin A1: roles in placenta, cell survival, and nucleus. Cells. 2022;11(13):2057. https://doi.org/10.3390/cells11132057
Xu X, Gao W, Li L, Hao J, Yang B, Wang T, et al. Annexin A1 protects against cerebral ischemia-reperfusion injury by modulating microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR pathway. J Neuroinflammation. 2021;18(1):119.
https://doi.org/10.1186/s12974-021-02174-3
You Q, Ke Y, Chen X, Yan W, Li D, Chen L, et al. Loss of endothelial annexin A1 aggravates inflammation-induced vascular aging. Adv Sci (Weinh). 2024;11(15):e2307040.
https://doi.org/10.1002/advs.202307040
Li C, Yu J, Liao D, Su X, Yi X, Yang X, et al. Annexin A2: the missing piece in the puzzle of pathogen-induced damage. Virulence. 2023;14(1):2237222. https://doi.org/10.1080/21505594.2023.2237222
Wang T, Zhao D, Zhang Y, Yu D, Liu G, Zhang K. Annexin A2: a double-edged sword in pathogen infection. Pathogens. 2024;13(7):564. https://doi.org/10.3390/pathogens13070564
Christofidis K, Pergaris A, Fioretzaki R, Charalampakis N, Kapetanakis EΙ, Kavantzas N, et al. Annexin A2 in tumors of the gastrointestinal tract, liver, and pancreas. Cancers (Basel). 2024;16(22):3764. https://doi.org/10.3390/cancers16223764
Luo M, Almeida D, Dallacasagrande V, Hedhli N, Gupta M, D'Amico DJ, et al. Annexin A2 promotes proliferative vitreoretinopathy in response to a macrophage inflammatory signal in mice. Nat Commun. 2024;15(1):8757. https://doi.org/10.1038/s41467-024-52675-x
Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J, et al. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med. 2005;46(12):2035–50.
Jin M, Zhang J, Sun Y, Liu G, Wei X. ANXA5: related mechanisms of osteogenesis and additional biological functions. Front Cell Dev Biol. 2025;13:1553683.
https://doi.org/10.3389/fcell.2025.1553683
Jing J. The relevance, predictability, and utility of annexin A5 for human physiopathology. Int J Mol Sci. 2024;25(5):2865. https://doi.org/10.3390/ijms25052865
Murad H, Ali B, Twair A, Baghdadi K, Alhalabi M, Abbady AQ. “In House” assays for the quantification of Annexin V and its autoantibodies in patients with recurrent pregnancy loss and in vitro fertilisation failures. Sci Rep. 2023;13(1):22322.
https://doi.org/10.1038/s41598-023-49768-w
Siegel CH, Sammaritano LR. Systemic lupus erythematosus: a review. JAMA. 2024;331(17):1480–91.
https://doi.org/10.1001/jama.2024.2315
Tian J, Zhang D, Yao X, Huang Y, Lu Q. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis. 2023;82(3):351–56.
https://doi.org/10.1136/ard-2022-223035
Ameer MA, Chaudhry H, Mushtaq J, Khan OS, Babar M, Hashim T, et al. An overview of systemic lupus erythematosus (SLE) pathogenesis, classification, and management. Cureus. 2022;14(10):e30330.
https://doi.org/10.7759/cureus.30330
Su X, Yu H, Lei Q, Chen X, Tong Y, Zhang Z, et al. Systemic lupus erythematosus: pathogenesis and targeted therapy. Mol Biomed. 2024;5(1):54. https://doi.org/10.1186/s43556-024-00217-8
Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9(1):62–70. https://doi.org/10.1038/nri2470
Li Y, Xu B, Zhang J, Liu X, Ganesan K, Shi G. Exploring the role of LIAS- related cuproptosis in systemic lupus erythematosus. Lupus. 2023;32(14):1598– 609.
https://doi.org/10.1177/09612033231211429
Mihaylova N, Chipinski P, Bradyanova S, Velikova T, Ivanova-Todorova E, Chausheva S, et al. Suppression of autoreactive T and B lymphocytes by anti- annexin A1 antibody in a humanized NSG murine model of systemic lupus erythematosus. Clin Exp Immunol. 2020;199(3):278–93. https://doi.org/10.1111/cei.13399
Dhaffouli F, Hachicha H, Abida O, Gharbi N, Elloumi N, Kanoun H, et al. Annexin A1 and its receptor gene polymorphisms in systemic lupus erythematosus in the Tunisian population. Clin Rheumatol. 2022;41(5):1359–69. https://doi.org/10.1007/s10067-022-06057-7
Parikh SV, Almaani S, Brodsky S, Rovin BH. Update on lupus nephritis: Core Curriculum 2020. Am J Kidney Dis. 2020;76(2):265–81. https://doi.org/10.1053/j.ajkd.2019.10.017
Hoi A, Igel T, Mok CC, Arnaud L. Systemic lupus erythematosus. Lancet. 2024;403(10441):2326–38.
https://doi.org/10.1016/S0140-6736(24)00398-2
Roveta A, Parodi EL, Brezzi B, Tunesi F, Zanetti V, Merlotti G, et al. Lupus nephritis from pathogenesis to new therapies: an update. Int J Mol Sci. 2024;25(16):8981.
https://doi.org/10.3390/ijms25168981
Tsai CY, Li KJ, Shen CY, Lu CH, Lee HT, Wu TH, et al. Decipher the immunopathological mechanisms and set up potential therapeutic strategies for patients with lupus nephritis. Int J Mol Sci. 2023;24(12):10066. https://doi.org/10.3390/ijms241210066
Yung S, Cheung KF, Zhang Q, Chan TM. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J Am Soc Nephrol. 2010;21(11):1912–27. https://doi.org/10.1681/ASN.2009080805
Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. 2019;393(10188):2344–58.
https://doi.org/10.1016/S0140-6736(19)30546-X
Tektonidou MG, Lewandowski LB, Hu J, Dasgupta A, Ward MM. Survival in adults and children with systemic lupus erythematosus: a systematic review and Bayesian meta-analysis of studies from 1950 to 2016. Ann Rheum Dis. 2017;76(12):2009–16.
https://doi.org/10.1136/annrheumdis-2017-211663
Frostegård J. Systemic lupus erythematosus and cardiovascular disease. J Intern Med. 2023;293(1):48–62.
https://doi.org/10.1111/joim.13557
Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376(9751):1498–509.
https://doi.org/10.1016/S0140-6736(10)60709-X
Cederholm A, Svenungsson E, Jensen-Urstad K, Trollmo C, Ulfgren AK, Swedenborg J, et al. Decreased binding of annexin V to endothelial cells: a potential mechanism in atherothrombosis of patients with systemic lupus erythematosus. Arterioscler Thromb Vasc Biol. 2005;25(1):198–203. https://doi.org/10.1161/01.ATV.0000150415.18759.36
Su J, Frostegård AG, Hua X, Gustafsson T, Jogestrand T, Hafström I, et al. Low levels of antibodies against oxidized but not nonoxidized cardiolipin and phosphatidylserine are associated with atherosclerotic plaques in systemic lupus erythematosus. J Rheumatol. 2013;40(11):1856–64. https://doi.org/10.3899/jrheum.121173
Cederholm A, Frostegård J. Annexin A5 as a novel player in prevention of atherothrombosis in SLE and in the general population. Ann N Y Acad Sci. 2007;1108:96–103.
https://doi.org/10.1196/annals.1422.011
Wan M, Hua X, Su J, Thiagarajan D, Frostegård AG, Haeggström JZ, et al. Oxidized but not native cardiolipin has pro-inflammatory effects, which are inhibited by Annexin A5. Atherosclerosis. 2014;235(2):592–98. https://doi.org/10.1016/j.atherosclerosis.2014.05.913
Knight JS, Branch DW, Ortel TL. Antiphospholipid syndrome: advances in diagnosis, pathogenesis, and management. BMJ. 2023;380:e069717. https://doi.org/10.1136/bmj-2021-069717
Duarte-García A, Pham MM, Crowson CS, Amin S, Moder KG, Pruthi RK, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71(9):1545–52.
https://doi.org/10.1002/art.40901
Dabit JY, Valenzuela-Almada MO, Vallejo-Ramos S, Duarte-García A. Epidemiology of antiphospholipid syndrome in the general population. Curr Rheumatol Rep. 2022;23(12):85.
https://doi.org/10.1007/s11926-021-01038-2
Brownstein C, Deora AB, Jacovina AT, Weintraub R, Gertler M, Khan KM, et al. Annexin II mediates plasminogen-dependent matrix invasion by human monocytes: enhanced expression by macrophages. Blood. 2004;103(1):317–24. https://doi.org/10.1182/blood-2003-04-1304
Cesarman-Maus G, Cantú-Brito C, Barinagarrementeria F, Villa R, Reyes E, Sanchez-Guerrero J, et al. Autoantibodies against the fibrinolytic receptor, annexin A2, in cerebral venous thrombosis. Stroke. 2011;42(2):501–3. https://doi.org/10.1161/STROKEAHA.110.592121
Green D. Pathophysiology of antiphospholipid syndrome. Thromb Haemost. 2022;122(7):1085–95.
https://doi.org/10.1055/a-1701-2809
Antovic A, Bruzelius M. Impaired fibrinolysis in the antiphospholipid syndrome. Semin Thromb Hemost. 2021;47(5):506–11.
https://doi.org/10.1055/s-0041-1725098
Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2005;105(5):1964– 9.
https://doi.org/10.1182/blood-2004-05-1708
Rand JH. Antiphospholipid antibody-mediated disruption of the annexin-V antithrombotic shield: a thrombogenic mechanism for the antiphospholipid syndrome. J Autoimmun. 2000;15(2):107–11. https://doi.org/10.1006/jaut.2000.0410
Roselli D, Bonifacio MA, Barbuti G, Rossiello MR, Ranieri P, Mariggiò MA. Anti-phosphatidylserine, anti-prothrombin, and anti-annexin V autoantibodies in antiphospholipid syndrome: a real-life study. Diagnostics (Basel). 2023;13(15):2507.
https://doi.org/10.3390/diagnostics13152507
Alijotas-Reig J, Esteve-Valverde E, Anunciación-Llunell A, Marques-Soares J, Pardos-Gea J, Miró-Mur F. Pathogenesis, diagnosis and management of obstetric antiphospholipid syndrome: a comprehensive review. J Clin Med. 2022;11(3):675. https://doi.org/10.3390/jcm11030675
Mekinian A, Alijotas-Reig J, Carrat F, Costedoat-Chalumeau N, Ruffatti A, Lazzaroni MG, et al. Refractory obstetrical antiphospholipid syndrome: features, treatment and outcome in a European multicenter retrospective study. Autoimmun Rev. 2017;16(7):730–34. https://doi.org/10.1016/j.autrev.2017.05.006
Di Simone N, Castellani R, Caliandro D, Caruso A. Monoclonal anti-annexin V antibody inhibits trophoblast gonadotropin secretion and induces syncytiotrophoblast apoptosis. Biol Reprod. 2001;65(6):1766–70. https://doi.org/10.1095/biolreprod65.6.1766
Di Matteo A, Bathon JM, Emery P. Rheumatoid arthritis. Lancet. 2023;402(10416):2019–33.
https://doi.org/10.1016/S0140-6736(23)01525-8
Gao Y, Zhang Y, Liu X. Rheumatoid arthritis: pathogenesis and therapeutic advances. MedComm (2020). 2024;5(3):e509. https://doi.org/10.1002/mco2.509
Ding Q, Hu W, Wang R, Yang Q, Zhu M, Li M, et al. Signaling pathways in rheumatoid arthritis: implications for targeted therapy. Signal Transduct Target Ther. 2023;8(1):68.
https://doi.org/10.1038/s41392-023-01331-9
Yang YH, Morand E, Leech M. Annexin A1: potential for glucocorticoid sparing in RA. Nat Rev Rheumatol. 2013;9(10):595–603. https://doi.org/10.1038/nrrheum.2013.126
Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. IL-6 stimulates annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine. 1998;10(7):514–21. https://doi.org/10.1006/cyto.1997.0325
Yanding G, Kun L, Linlin Z, Wenting LU, Yanan S, Yumei Z, et al. Study on the anti-inflammatory mechanism of moxibustion in rheumatoid arthritis in rats based on phospholipaseA2 signaling inhibition by Annexin 1. J Tradit Chin Med. 2024;44(4):753–61.
Chen J, Norling LV, Mesa JG, Silva MP, Burton SE, Reutelingsperger C, et al. Annexin A1 attenuates cardiac diastolic dysfunction in mice with inflammatory arthritis. Proc Natl Acad Sci U S A. 2021;118(38):e2020385118. https://doi.org/10.1073/pnas.2020385118
Wang J, Zhao J, Lin L, Peng X, Li W, Huang Y, et al. LncRNA-Anrel promotes the proliferation and migration of synovial fibroblasts through regulating miR- 146a-mediated annexin A1 expression. Am J Clin Exp Immunol. 2023;12(4):49– 59.
Tsen SD, Springer LE, Sharmah Gautam K, Tang R, Liang K, Sudlow G, et al. Non-invasive monitoring of arthritis treatment response via targeting of tyrosine- phosphorylated annexin A2 in chondrocytes. Arthritis Res Ther. 2021;23(1):265. https://doi.org/10.1186/s13075-021-02643-3
Yi J, Zhu Y, Jia Y, Jiang H, Zheng X, Liu D, et al. The Annexin A2 promotes development in arthritis through neovascularization by amplification Hedgehog pathway. PLoS One. 2016;11(3):e0150363. https://doi.org/10.1371/journal.pone.0150363
Xiao S, Ouyang Q, Feng Y, Lu X, Han Y, Ren H, et al. LncNFYB promotes the proliferation of rheumatoid arthritis fibroblast-like synoviocytes via LncNFYB/ANXA2/ERK1/2 axis. J Biol Chem. 2024;300(2):105591. https://doi.org/10.1016/j.jbc.2023.105591
Zhao W, Zhang C, Shi M, Zhang J, Li M, Xue X, et al. The discoidin domain receptor 2/annexin A2/matrix metalloproteinase 13 loop promotes joint destruction in arthritis through promoting migration and invasion of fibroblast- like synoviocytes. Arthritis Rheumatol. 2014;66(9):2355–67. https://doi.org/10.1002/art.38696
Yin G, Yang C, Wu G, Yu X, Tian Q, Chen D, et al. The protein-protein interaction between connective tissue growth factor and annexin A2 is relevant to pannus formation in rheumatoid arthritis. Arthritis Res Ther. 2021;23(1):266. https://doi.org/10.1186/s13075-021-02656-y
Baldini C, Fulvio G, La Rocca G, Ferro F. Update on the pathophysiology and treatment of primary Sjögren syndrome. Nat Rev Rheumatol. 2024;20(8):473–91. https://doi.org/10.1038/s41584-024-01135-3
Liang Y, Yang Z, Qin B, Zhong R. Primary Sjogren's syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis. 2014;73(6):1151–6. https://doi.org/10.1136/annrheumdis-2013-203305
Cui L, Elzakra N, Xu S, Xiao GG, Yang Y, Hu S. Investigation of three potential autoantibodies in Sjogren's syndrome and associated MALT lymphoma. Oncotarget. 2017;8(18):30039–49.
https://doi.org/10.18632/oncotarget.15613
Finamore F, Cecchettini A, Ceccherini E, Signore G, Ferro F, Rocchiccioli S, et al. Characterization of extracellular vesicle cargo in Sjögren's syndrome through a SWATH-MS proteomics approach. Int J Mol Sci. 2021;22(9):4864. https://doi.org/10.3390/ijms22094864
Peck AB, Ambrus JL Jr. A temporal comparative RNA transcriptome profile of the annexin gene family in the salivary versus lacrimal glands of the Sjögren's syndrome-susceptible C57BL/6.NOD-Aec1Aec2 mouse. Int J Mol Sci. 2022;23(19):11709.
https://doi.org/10.3390/ijms231911709
McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325(8):765–79. https://doi.org/10.1001/jama.2020.26858
White ZB 2nd, Nair S, Bredel M. The role of annexins in central nervous system development and disease. J Mol Med (Berl). 2024;102(6):751–60. https://doi.org/10.1007/s00109-024-02443-7
Hejazi MS, Jafari S, Montazersaheb S, Molavi O, Hosseini V, Talebi M, et al. Annexin A1, calreticulin and high mobility group box 1 are elevated in secondary progressive multiple sclerosis: does immunogenic cell death occur in multiple sclerosis? Bioimpacts. 2024;15:30264.
https://doi.org/10.34172/bi.30264
Tezuka K, Suzuki M, Sato R, Kawarada S, Terasaki T, Uchida Y. Activation of annexin A2 signaling at the blood-brain barrier in a mouse model of multiple sclerosis. J Neurochem. 2022;160(6):662–74. https://doi.org/10.1111/jnc.15578
Bruschi M, Moroni G, Sinico RA, Franceschini F, Fredi M, Vaglio A, et al. Serum IgG2 antibody multicomposition in systemic lupus erythematosus and lupus nephritis (Part 1): cross-sectional analysis. Rheumatology (Oxford). 2021;60(7):3176–88.
https://doi.org/10.1093/rheumatology/keaa767
Bruschi M, Moroni G, Sinico RA, Franceschini F, Fredi M, Vaglio A, et al. Serum IgG2 antibody multi-composition in systemic lupus erythematosus and in lupus nephritis (Part 2): prospective study. Rheumatology (Oxford). 2021;60(7):3388–97.
https://doi.org/10.1093/rheumatology/keaa793
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295– 306.
https://doi.org/10.1111/j.1538-7836.2006.01753.x
Jawad AS. Seronegative antiphospholipid syndrome. Ann Rheum Dis. 2004;63(5):608.
Sciascia S, Amigo MC, Roccatello D, et al. Diagnosing antiphospholipid syndrome: “extra-criteria” manifestations and technical advances. Nat Rev Rheumatol. 2017;13(9):548–60.
https://doi.org/10.1038/nrrheum.2017.124
Bradacova P, Slavik L, Ulehlova J, Skoumalova A, Ullrychova J, Prochazkova J, et al. Current promising biomarkers and methods in the diagnostics of antiphospholipid syndrome: a review. Biomedicines. 2021;9(2):166. https://doi.org/10.3390/biomedicines9020166
Cañas F, Simonin L, Couturaud F, Renaudineau Y. Annexin A2 autoantibodies in thrombosis and autoimmune diseases. Thromb Res. 2015;135(2):226–30. https://doi.org/10.1016/j.thromres.2014.11.034
Cesarman-Maus G, Ríos-Luna NP, Deora AB, Huang B, Villa R, Cravioto Mdel C, et al. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood. 2006;107(11):4375–82. https://doi.org/10.1182/blood-2005-07-2636
Ao W, Zheng H, Chen XW, Shen Y, Yang CD. Anti-annexin II antibody is associated with thrombosis and/or pregnancy morbidity in antiphospholipid syndrome and systemic lupus erythematosus with thrombosis. Rheumatol Int. 2011;31(7):865–9.
https://doi.org/10.1007/s00296-010-1379-4
Weiss R, Bushi D, Mindel E, Bitton A, Diesendruck Y, Gera O, et al. Autoantibodies to Annexin A2 and cerebral thrombosis: insights from a mouse model. Lupus. 2021;30(5):775–84. https://doi.org/10.1177/0961203321992117
Volkmann ER, Andréasson K, Smith V. Systemic sclerosis. Lancet. 2023;401(10373):304–18.
https://doi.org/10.1016/S0140-6736(22)01692-0
Sugiura K, Muro Y. Anti-annexin V antibodies and digital ischemia in patients with scleroderma. J Rheumatol. 1999;26(10):2168–72.
Esposito G, Tamby MC, Chanseaud Y, Servettaz A, Guillevin L, Mouthon L. Anti-annexin V antibodies: are they prothrombotic? Autoimmun Rev. 2005;4(1):55–60.
https://doi.org/10.1016/j.autrev.2004.07.006
El Serougy IM, Shahin AA, Soliman DA, Akhnoukh AF, Mousa SM. Clinical significance of serum anti-annexin V antibodies in Egyptian patients with scleroderma. Egypt J Immunol. 2009;16(1):1–8.
Horimoto AMC, de Jesus LG, de Souza AS, Rodrigues SH, Kayser C. Anti- annexin V autoantibodies and vascular abnormalities in systemic sclerosis: a longitudinal study. Adv Rheumatol. 2020;60(1):38. https://doi.org/10.1186/s42358-020-00140-w
Conti F, Andreoli L, Crisafulli F, Mancuso S, Truglia S, Tektonidou MG. Does seronegative obstetric APS exist? “pro” and “cons”. Autoimmun Rev. 2019;18(12):102407.
https://doi.org/10.1016/j.autrev.2019.102407
Ortona E, Capozzi A, Colasanti T, Conti F, Alessandri C, Longo A, et al. Vimentin/cardiolipin complex as a new antigenic target of the antiphospholipid syndrome. Blood. 2010;116(16):2960–7.
https://doi.org/10.1182/blood-2010-04-279208
Zhang S, Wu Z, Li J, Wen X, Li L, Zhang W, et al. Evaluation of the clinical relevance of anti-annexin-A5 antibodies in Chinese patients with antiphospholipid syndrome. Clin Rheumatol. 2017;36(2):407–12. https://doi.org/10.1007/s10067-016-3510-8
Lakos G, Kiss E, Regeczy N, Tarjan P, Soltesz P, Zeher M, et al.
Antiprothrombin and antiannexin V antibodies imply risk of thrombosis in patients with systemic autoimmune diseases. J Rheumatol. 2000;27(4):924–9.
Bradáčová P, Slavík L, Skoumalová A, Úlehlová J, Kriegová E, Manukyan G, et al. Determination of thrombogenicity levels of various antiphospholipid antibodies by a modified thrombin generation assay in patients with suspected antiphospholipid syndrome. Int J Mol Sci. 2022;23(16):8973. https://doi.org/10.3390/ijms23168973
Matsubayashi H, Arai T, Izumi S, Sugi T, McIntyre JA, Makino T. Anti-annexin V antibodies in patients with early pregnancy loss or implantation failures. Fertil Steril. 2001;76(4):694–9.
https://doi.org/10.1016/S0015-0282(01)02009-X
Xiong Y, Wu T, Wang L, Shen X, Yin Y, Wu J, et al. The clinical significance of non-criteria antiphospholipid antibodies in atypical antiphospholipid syndrome.
Mod Rheumatol. 2025;35(6):1009–14. https://doi.org/10.1093/mr/roaf060
Gris JC, Quéré I, Sanmarco M, Boutiere B, Mercier E, Amiral J, et al.
Antiphospholipid and antiprotein syndromes in non-thrombotic, non-autoimmune women with unexplained recurrent primary early foetal loss. The Nîmes Obstetricians and Haematologists Study–NOHA. Thromb Haemost.
;84(2):228–36.
https://doi.org/10.1055/s-0037-1614001
Guo X, Xiang J, Zhang W, Zheng X, Dai Y, Cai Z. Association of anti-annexin A5 antibody with pregnancy outcomes: a cohort study: Anti-annexin A5 antibody with pregnancy outcomes. Am J Reprod Immunol. 2024;92(4):e13936. https://doi.org/10.1111/aji.13936
Nasef A, Ibrahim M, Riad N, Mousa S. Plasma annexin A5, anti-annexin A5 antibodies and annexin A5 polymorphism in Egyptian female patients with systemic lupus erythematosus and antiphospholipid syndrome. Clin Lab.
;60(1):133–7.
https://doi.org/10.7754/Clin.Lab.2013.130112
Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Sáez-Comet L, Lefkou E, Mekinian A, et al. The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): a survey of 1000 consecutive cases. Autoimmun Rev. 2019;18(4):406–14.
https://doi.org/10.1016/j.autrev.2018.12.006
Bećarević M. The IgG and IgM isotypes of anti-annexin A5 antibodies: relevance for primary antiphospholipid syndrome. J Thromb Thrombolysis.
;42(4):552–7.
https://doi.org/10.1007/s11239-016-1389-5
Jiang S, Li H, Zhang L, Mu W, Zhang Y, Chen T, et al. Generic Diagramming Platform (GDP): a comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025;53(D1):D1670–D1676.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2026 Xiuli Zhou, Jinle Liu, Siyi Wang, Yexiao Zhang, Linjie Xu, Lan Wu

This work is licensed under a Creative Commons Attribution 4.0 International License.









