Pretreatment comprehensive nutritional index predicts survival in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery
DOI:
https://doi.org/10.17305/bb.2026.13609Keywords:
Comprehensive nutritional index, nutritional status, neoadjuvant chemoradiotherapy, prognosis, locally advanced rectal cancerAbstract
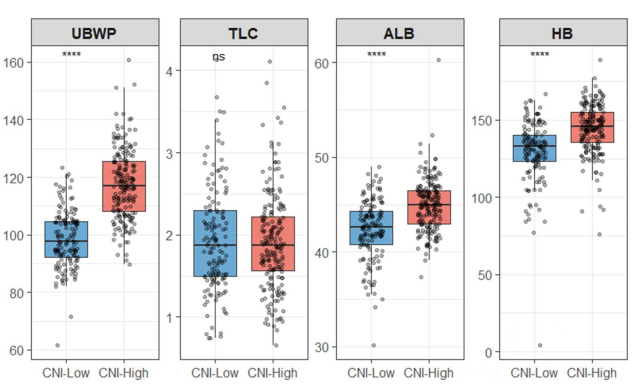
Nutritional status significantly influences treatment tolerance and long-term outcomes in patients with locally advanced rectal cancer (LARC); however, individual nutritional markers may not fully capture overall nutritional reserves. This study aimed to evaluate the prognostic value of a comprehensive nutritional index (CNI), derived from principal component analysis, in patients with LARC undergoing neoadjuvant chemoradiotherapy (NCRT) followed by surgical intervention. We conducted a retrospective analysis of 336 patients with LARC who received NCRT followed by surgery between 2014 and 2019. The CNI was constructed using body mass index, usual body weight percentage, total lymphocyte count, serum albumin, and hemoglobin levels. Patients were categorized into low- and high-CNI groups based on an outcome-oriented cut point, and survival outcomes were assessed through Kaplan–Meier analysis and Cox regression. Patients with lower CNI scores exhibited significantly poorer overall survival and disease-free survival compared to those with higher CNI scores. Furthermore, CNI remained independently associated with both endpoints after adjusting for established pathological factors, including tumor regression grade and ypN stage. A nomogram that integrates CNI, tumor regression grade, and ypN stage demonstrated favorable discrimination and calibration during internal validation. These findings support the use of pretreatment CNI as a practical nutritional composite associated with prognosis in LARC patients treated with NCRT, and the proposed nomogram may enhance individualized risk estimation.
Citations
Downloads
References
Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–54.
https://doi.org/10.3322/caac.21772
Koukourakis IM, Kouloulias V, Tiniakos D, Georgakopoulos I, Zygogianni A. Current status of locally advanced rectal cancer therapy and future prospects. Crit Rev Oncol Hematol. 2023;186:103992.
https://doi.org/10.1016/j.critrevonc.2023.103992
Smith HG, Nilsson PJ, Shogan BD, Harji D, Gambacorta MA, Romano A, et al. Neoadjuvant treatment of colorectal cancer: comprehensive review. BJS Open. 2024;8(3):zrae038.
https://doi.org/10.1093/bjsopen/zrae038
Scott AJ, Kennedy EB, Berlin J, Brown G, Chalabi M, Cho MT, et al. Management of locally advanced rectal cancer: ASCO guideline. J Clin Oncol. 2024;42(28):3355–75.
https://doi.org/10.1200/JCO.24.01160
Gaedcke J, Sahrhage M, Ebeling M, Azizian A, Rühlmann F, Bernhardt M, et al. Prognosis and quality of life in patients with locally advanced rectal cancer after abdominoperineal resection in the CAO/ARO/AIO-04 randomized phase 3 trial. Sci Rep. 2025;15(1):5401.
https://doi.org/10.1038/s41598-024-83105-z
Zhao R, Shen W, Zhao W, Peng W, Wan L, Chen S, et al. Integrating radiomics, pathomics, and biopsy-adapted immunoscore for predicting distant metastasis in locally advanced rectal cancer. ESMO Open. 2025;10(3):104102.
https://doi.org/10.1016/j.esmoop.2024.104102
Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg. 2019;43(3):659–95.
https://doi.org/10.1007/s00268-018-4844-y
Shao Z, Zou Y, Zou C, Xu Y. Nutritional indicators as predictive and prognostic biomarkers for neoadjuvant chemoradiotherapy outcomes in patients with locally advanced rectal cancer. Support Care Cancer. 2025;33(10):834.
https://doi.org/10.1007/s00520-025-09911-x
Christina NM, Tjahyanto T, Lie JG, Santoso TA, Albertus H, Octavianus D, et al. Hypoalbuminemia and colorectal cancer patients: any correlation?: a systematic review and meta-analysis. Medicine (Baltimore). 2023;102(8):e32938.
https://doi.org/10.1097/MD.0000000000032938
Shibutani M, Kashiwagi S, Fukuoka T, Iseki Y, Kasashima H, Maeda K. Impact of preoperative nutritional status on long-term survival in patients with stage I–III colorectal cancer. In Vivo. 2023;37(4):1765–74.
https://doi.org/10.21873/invivo.13265
Feng J, Wang L, Yang X, Chen Q, Cheng X. Comprehensive nutritional index predicts clinical outcomes for esophageal squamous cell carcinoma receiving neoadjuvant immunotherapy combined with chemotherapy. Int Immunopharmacol. 2023;121:110459.
https://doi.org/10.1016/j.intimp.2023.110459
Duan YY, Deng J, Su DF, Li WQ, Han Y, Li ZX, et al. Construction of a comprehensive nutritional index and comparison of its prognostic performance with the PNI and NRI for survival in older patients with nasopharyngeal carcinoma: a retrospective study. Support Care Cancer. 2021;29(9):5371–81.
https://doi.org/10.1007/s00520-021-06128-6
Deng J, He Y, Sun XS, Li JM, Xin MZ, Li WQ, et al. Construction of a comprehensive nutritional index and its correlation with quality of life and survival in patients with nasopharyngeal carcinoma undergoing IMRT: a prospective study. Oral Oncol. 2019;98:62–8.
https://doi.org/10.1016/j.oraloncology.2019.09.014
He Y, Chen L, Chen L, Hu W, Wang C, Tang L, et al. Relationship between the comprehensive nutritional index and the EORTC QLQ-H&N35 in nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Nutr Cancer. 2017;69(3):436–43.
https://doi.org/10.1080/01635581.2017.1283422
Xu Y, Shen P, Zhu J, Qian D, Gu K, Mao Y, et al. Comprehensive malnutritional index for predicting clinical outcomes in locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. Biomol Biomed. 2025;25(5):1079–91.
https://doi.org/10.17305/bb.2024.11188
Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12(1):19–23.
https://doi.org/10.1007/s003840050072
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48.
https://doi.org/10.1016/j.clnu.2016.07.015
Barao K, Abe Vicente Cavagnari M, Silva Fucuta P, Manoukian Forones N. Association between nutrition status and survival in elderly patients with colorectal cancer. Nutr Clin Pract. 2017;32(5):658–63.
https://doi.org/10.1177/0884533617706894
Antasouras G, Papadopoulou SK, Tolia M, Pandi AL, Spanoudaki M, Tsoukalas N, et al. May nutritional status positively affect disease progression and prognosis in patients with esophageal and pharyngeal cancers? A scoping review of the current clinical studies. Med Sci (Basel). 2023;11(4):64.
https://doi.org/10.3390/medsci11040064
Deng J, He JJ, Xie LP, Zheng QW, Wu SL, Shao HY. Comprehensive nutritional index for predicting overall survival in hepatocellular carcinoma patients after multiple transarterial chemoembolization. Asia Pac J Clin Nutr. 2021;30(1):7–14.
https://doi.org/10.6133/apjcn.202103_30(1).0002
Kaluźniak-Szymanowska A, Krzymińska-Siemaszko R, Deskur-Śmielecka E, Lewandowicz M, Kaczmarek B, Wieczorowska-Tobis K. Malnutrition, sarcopenia, and malnutrition-sarcopenia syndrome in older adults with COPD. Nutrients. 2022;14(1):44.
https://doi.org/10.3390/nu14010044
Xu Y, Zhao Y, Wang J, Gao S, Sun Q, Ali M, et al. Association of preoperative nutritional status with sarcopenia in patients with gastrointestinal malignancies assessed by Global Leadership Initiative in Malnutrition criteria: a prospective cohort study. Ann Nutr Metab. 2025;81(2):68–79.
https://doi.org/10.1159/000542698
Kim CH, Park SM, Kim JJ. The impact of preoperative low body mass index on postoperative complications and long-term survival outcomes in gastric cancer patients. J Gastric Cancer. 2018;18(3):274–86.
https://doi.org/10.5230/jgc.2018.18.e30
Goh SL, De Silva RP, Dhital K, Gett RM. Is low serum albumin associated with postoperative complications in patients undergoing oesophagectomy for oesophageal malignancies? Interact Cardiovasc Thorac Surg. 2015;20(1):107–13.
https://doi.org/10.1093/icvts/ivu324
Onji M, Kakizoe S, Naito K, Date K, Nakai A, Shimizu K, et al. Preoperative frailty as a predictive factor for postoperative complications in patients with pancreatic cancer. Nagoya J Med Sci. 2023;85(3):518–27.
https://doi.org/10.18999/nagjms.85.3.518
Ma XB, Lv YL, Qian L, Huang ST, Pu XX, Liu YM. Ratio of red blood cell distribution width to albumin level and risk of mortality in sarcopenic obesity. Sci Rep. 2024;14:27886.
https://doi.org/10.1038/s41598-024-79055-1
Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503.
https://doi.org/10.1016/S1470-2045(14)70263-3
Sim JH, Bang JY, Kim SH, Kang SJ, Song JG. Association of preoperative prognostic nutritional index and postoperative acute kidney injury in patients with colorectal cancer surgery. Nutrients. 2021;13(5):1604.
https://doi.org/10.3390/nu13051604
Li K, Yan J, Zhang H. Correlation between peripheral blood hemoglobin/erythrocyte distribution width ratio and prognosis of patients with primary colorectal cancer. Medicine (Baltimore). 2023;102(23):e34031.
https://doi.org/10.1097/MD.0000000000034031
Dagmura H, Daldal E, Okan I. The efficacy of hemoglobin, albumin, lymphocytes, and platelets as a prognostic marker for survival in octogenarians and nonagenarians undergoing colorectal cancer surgery. Cancer Biother Radiopharm. 2022;37(10):955–62.
https://doi.org/10.1089/cbr.2020.4725
Han Y, Zhou P, Wang L, Tang Y, Ding Y, Yang Y, et al. Linear inverse association between prognostic nutritional index and colorectal cancer risk based on NHANES data. Sci Rep. 2025;15(1):25647.
https://doi.org/10.1038/s41598-025-10574-1
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, et al. Impact of the preoperative prognostic nutritional index on postoperative and survival outcomes in colorectal cancer patients who underwent primary tumor resection: a systematic review and meta-analysis. Int J Colorectal Dis. 2019;34(4):681–9.
https://doi.org/10.1007/s00384-019-03241-1
Yuan J, Hu Y, Wang J, Chang L, Guan T, Fu X, et al. A model based on preoperative nutrition-inflammation score for predicting mucocutaneous separation after enterostomy in colorectal cancer patients. Sci Rep. 2025;15(1):31095.
https://doi.org/10.1038/s41598-025-16430-6
Xu R, Gong LF. Development and evaluation of a nomogram model for predicting malnutrition in patients with colorectal cancer. Front Med. 2025;12:1637579.
https://doi.org/10.3389/fmed.2025.1637579
Yin H, Yao Q, Xie Y, Niu D, Jiang W, Cao H, et al. Tumor regression grade combined with post-therapy lymph node status: a novel independent prognostic factor for patients treated with neoadjuvant therapy followed by surgery in locally advanced gastroesophageal junction and gastric carcinoma. Cancer Med. 2023;12(19):19633–43.
https://doi.org/10.1002/cam4.6597
Mendoza-Moreno F, Díez-Alonso M, Matías-García B, Ovejero-Merino E, Vera-Mansilla C, Quiroga-Valcárcel A, et al. Effect of tumor regression grade on survival and disease-free interval in patients operated on for locally advanced rectal cancer. Cancers (Basel). 2024;16(10):1797.
https://doi.org/10.3390/cancers16101797
Wiesmueller F, Schuetz R, Langheinrich M, Brunner M, Weber GF, Grützmann R, et al. Defining early recurrence in patients with resected primary colorectal carcinoma and its respective risk factors. Int J Colorectal Dis. 2021;36(6):1181–91.
https://doi.org/10.1007/s00384-021-03844-7
Mills MN, Naz A, Sanchez J, Dessureault S, Imanirad I, Lauwers G, et al. Rectal tumor fragmentation as a response pattern following chemoradiation. J Gastrointest Oncol. 2022;13(6):2951–62.
https://doi.org/10.21037/jgo-22-477
Kim HG, Kim HS, Yang SY, Han YD, Cho MS, Hur H, et al. Early recurrence after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: characteristics and risk factors. Asian J Surg. 2021;44(1):298–302.
https://doi.org/10.1016/j.asjsur.2020.07.014
Damanakis AI, Gebauer F, Stapper A, Schlößer HA, Ghadimi M, Schmidt T, et al. Combined regression score predicts outcome after neoadjuvant treatment of oesophageal cancer. Br J Cancer. 2023;128(11):2025–35.
https://doi.org/10.1038/s41416-023-02232-y
Yamano T, Yoshimura M, Kobayashi M, Beppu N, Hamanaka M, Babaya A, et al. Malnutrition in rectal cancer patients receiving preoperative chemoradiotherapy is common and associated with treatment tolerability and anastomotic leakage. Int J Colorectal Dis. 2016;31(4):877–84.
https://doi.org/10.1007/s00384-016-2507-8
Ide S, Okugawa Y, Omura Y, Yamamoto A, Ichikawa T, Kitajima T, et al. Geriatric nutritional risk index predicts cancer prognosis in patients with local advanced rectal cancer undergoing chemoradiotherapy followed by curative surgery. World J Surg Oncol. 2021;19:34.
https://doi.org/10.1186/s12957-021-02139-z
Aliyev V, Goksel S, Bakir B, Guven K, Asoglu O. Sphincter-saving robotic total mesorectal excision provides better mesorectal specimen and good oncological local control compared with laparoscopic total mesorectal excision in male patients with mid–low rectal cancer. Surg Technol Int. 2021;38:160–6.
https://doi.org/10.52198/21.STI.38.CR1391
Aliyev V, Piozzi GN, Huseynov E, Mustafayev TZ, Kayku V, Goksel S, et al. Robotic male and laparoscopic female sphincter-preserving total mesorectal excision of mid–low rectal cancer share similar specimen quality, complication rates and long-term oncological outcomes. J Robot Surg. 2023;17(4):1637–44.
https://doi.org/10.1007/s11701-023-01558-2
Aliyev V, Piozzi GN, Shadmanov N, Guven K, Bakır B, Goksel S, et al. Robotic and laparoscopic sphincter-saving resections have similar peri-operative, oncological and functional outcomes in female patients with rectal cancer. Updat Surg. 2023;75(8):2201–9.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2026 Zhexue Wang, Liming Zhao, Pu Cheng, Mandula Bao, Fei Huang, Ruoxi Tian, Jiyun Li, Hengchang Liu, Zhaoxu Zheng

This work is licensed under a Creative Commons Attribution 4.0 International License.









