Impact of active smoking on survival of patients with metastatic lung adenocarcinoma harboring an epidermal growth factor receptor (EGFR) mutation
DOI:
https://doi.org/10.17305/bjbms.2016.1380Keywords:
Lung adenocarcinoma, epidermal growth factor receptor, smokingAbstract
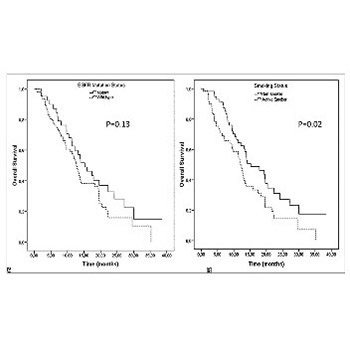
Lung cancer in smokers and non-smokers demonstrates distinct genetic profiles, and cigarette smoking affects epidermal growth factor receptor (EGFR) function and causes secondary EGFR tyrosine kinase resistance. We evaluated the effect of active smoking in patients with metastatic lung adenocarcinoma. A total of 132 metastatic lung adenocarcinoma patients, diagnosed between 2008 and 2013, with known EGFR mutation status, were evaluated retrospectively. Among these patients, 40 had an activating EGFR mutation. Patients who continued smoking during the treatment were defined as active smokers. Former smokers and never smokers were together defined as non-smokers. The outcomes of the treatment in relation to the EGFR mutation and smoking status were evaluated. The median follow-up time was 10.5 months. The overall response rate for the first-line therapy was significantly higher among the EGFR-mutant patients (p = 0.01), however, smoking status had no impact on the response rate (p = 0.1). The EGFR-mutant active smokers progressed earlier than the non-smokers (p < 0.01). The overall survival (OS) of the non-smokers and patients treated with erlotinib was significantly longer (p = 0.02 and p = 0.01, respectively). Smoking status did not affect the OS in EGFR wild type tumors (p = 0.49) but EGFR-mutant non-smokers had a longer OS than the active smokers (p = 0.01).The active smokers treated with erlotinib had poorer survival than the non-smokers (p = 0.03). Multivariate analysis of EGFR-mutant patients showed that erlotinib treatment at any line and non-smoking were independent prognostic factors for the OS (p = 0.04 and p = 0.01, respectively). Smoking during treatment is a negative prognostic factor in metastatic lung adenocarcinoma with an EGFR mutation.
Citations
Downloads
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63(1):11-30. http://dx.doi.org/10.3322/caac.21166.
Scagliotti GV, Longo M, Novello S. Nonsmall cell lung cancer in never smokers. Curr Opin Oncol 2009;21(2):99-104. http://dx.doi.org/10.1097/CCO.0b013e328321049e.
Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150(6):1121-34. http://dx.doi.org/10.1016/j.cell.2012.08.024.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353(2):123-32. http://dx.doi.org/10.1056/NEJMoa050753.
Park K, Goto K. A review of the benefit-risk profile of gefitinib in Asian patients with advanced non-small-cell lung cancer. Curr Med Res Opin 2006;22(3):561-73.
http://dx.doi.org/10.1185/030079906X89847.
Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 2004;64(24):8919-23. http://dx.doi.org/10.1158/0008-5472.CAN-04-2818.
Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba, II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97(5):339-46. http://dx.doi.org/10.1093/jnci/dji055.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350(21):2129-39.
http://dx.doi.org/10.1056/NEJMoa040938.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304(5676):1497-500. http://dx.doi.org/10.1126/science.1099314.
Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361(10):958-67. http://dx.doi.org/10.1056/NEJMoa0904554.
D'Angelo SP, Pietanza MC, Johnson ML, Riely GJ, Miller VA, Sima CS, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 2011;29(15):2066-70.
http://dx.doi.org/10.1200/JCO.2010.32.6181.
Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5(5):620-30. http://dx.doi.org/10.1097/JTO.0b013e3181d2dcd9.
Yoshino I, Maehara Y. Impact of smoking status on the biological behavior of lung cancer. Surg Today 2007;37(9):725-34. http://dx.doi.org/10.1007/s00595-007-3516-6.
O'Malley M, King AN, Conte M, Ellingrod VL, Ramnath N. Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer. J Thorac Oncol 2014;9(7):917-26. http://dx.doi.org/10.1097/JTO.0000000000000191.
Lee YJ, Shim HS, Kang YA, Hong SJ, Kim HK, Kim H, et al. Dose effect of cigarette smoking on frequency and spectrum of epidermal growth factor receptor gene mutations in Korean patients with non-small cell lung cancer. J Cancer Res Clin Oncol 2010;136(12):1937-44. http://dx.doi.org/10.1007/s00432-010-0853-4.
Zhang Q, Dai HH, Dong HY, Sun CT, Yang Z, Han JQ. EGFR mutations and clinical outcomes of chemotherapy for advanced non-small cell lung cancer: a meta-analysis. Lung Cancer 2014;85(3):339-45. http://dx.doi.org/10.1016/j.lungcan.2014.06.011.
Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105(9):595-605. http://dx.doi.org/10.1093/jnci/djt072.
Ferketich AK, Niland JC, Mamet R, Zornosa C, D'Amico TA, Ettinger DS, et al. Smoking status and survival in the national comprehensive cancer network non-small cell lung cancer cohort. Cancer 2013;119(4):847-53. http://dx.doi.org/10.1002/cncr.27824.
Filosto S, Becker CR, Goldkorn T. Cigarette smoke induces aberrant EGF receptor activation that mediates lung cancer development and resistance to tyrosine kinase inhibitors. Mol Cancer Ther 2012;11(4):795-804. http://dx.doi.org/10.1158/1535-7163.MCT-11-0698.
Filosto S, Baston DS, Chung S, Becker CR, Goldkorn T. Src mediates cigarette smoke-induced resistance to tyrosine kinase inhibitors in NSCLC cells. Mol Cancer Ther 2013;12(8):1579-90. http://dx.doi.org/10.1158/1535-7163.MCT-12-1029.
Hamilton M, Wolf JL, Rusk J, Beard SE, Clark GM, Witt K, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res 2006;12(7 Pt 1):2166-71.
http://dx.doi.org/10.1158/1078-0432.CCR-05-2235.
Lu JF, Eppler SM, Wolf J, Hamilton M, Rakhit A, Bruno R, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther 2006;80(2):136-45.
http://dx.doi.org/10.1016/j.clpt.2006.04.007.
Kim MH, Kim HR, Cho BC, Bae MK, Kim EY, Lee CY, et al. Impact of cigarette smoking on response to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations. Lung Cancer 2014;84(2):196-202.
http://dx.doi.org/10.1016/j.lungcan.2014.01.022.
Mitchell P, Mok T, Barraclough H, Strizek A, Lew R, van Kooten M. Smoking history as a predictive factor of treatment response in advanced non-small-cell lung cancer: a systematic review. Clin Lung Cancer 2012;13(4):239-51.
http://dx.doi.org/10.1016/j.cllc.2011.08.003.
Zhang Y, Kang S, Fang W, Hong S, Liang W, Yan Y, et al. Impact of smoking status on EGFR-TKI efficacy for advanced non-small-cell lung cancer in EGFR mutants: a meta-analysis. Clin Lung Cancer 2015;16(2):144-151.e1.http://dx.doi.org/10.1016/j.lungcan.2014.06.011.
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372(9652):1809-18. http://dx.doi.org/10.1016/S0140-6736(08)61758-4.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11(2):121-8.
http://dx.doi.org/10.1016/S1470-2045(09)70364-X.
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12(8):735-42. http://dx.doi.org/10.1016/S1470-2045(11)70184-X.
Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit. 2009;31(1):14-30.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-06-16
Published 2016-11-10









