A comparison of blood and cerebrospinal fluid cytokines (IL-1β, IL-6, IL-18, TNF-α) in neonates with perinatal hypoxia
DOI:
https://doi.org/10.17305/bjbms.2017.1381Keywords:
Neonates, cytokines, perinatal hypoxia, hypoxic-ischemic encephalopathy, neurological outcome, IL-1β, IL-6, IL-18, TNF-αAbstract
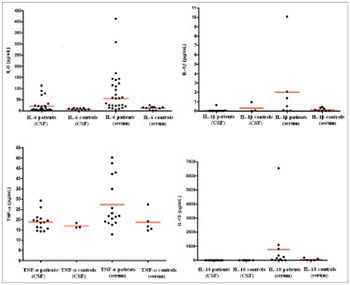
Perinatal hypoxia-ischemia is a specific and important pathological event in neonatal care practice. The data on relationship between the concentrations of cytokines in blood and cerebrospinal fluid (CSF) and perinatal brain injury are scarce. The aim of this study is to evaluate changes in interleukin (IL-1β, IL-6, and IL-18) and tumor necrosis factor alpha (TNF-α) levels in newborns with perinatal hypoxia (PNH). CSF and serum samples of 35 term and near-term (35-40 weeks) newborns with PNH, at the age of 3-96 hours, were analyzed using enzyme-linked immunosorbent assay. Control group consisted of 25 non-asphyxic/non-hypoxic infants of the same age sampled for clinically suspected perinatal meningitis, but proven negative and healthy otherwise. The cytokine values in CSF and serum samples were determined in relation to initial hypoxic-ischemic encephalopathy (HIE) staged according the Sarnat/Sarnat method, and compared with neurological outcome at 12 months of age estimated using Amiel-Tison procedure. The concentrations of IL-6 and TNF-α in serum of PNH patients were significantly higher compared to control group (p = 0.0407 and p = 0.023, respectively). No significant difference between average values of cytokines in relation to the stage of HIE was observed. Significantly higher levels of IL-6 and IL-18 corresponded to a mildly abnormal neurological outcome, while higher levels of IL-6 and TNF-α corresponded to a severely abnormal neurological outcome, at 12 months of age. Elevated serum levels of IL-6 and TNF-α better corresponded with hypoxia/ischemia compared to CSF values, within 96 hours of birth. Also, higher serum levels of IL-6, TNF-α, and IL-18 corresponded better with abnormal neurological outcome at 12 months of age, compared to CSF values.
Citations
Downloads
References
Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, et al. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatr 1997;2(2):133-6. https://doi.org/10.1038/sj.mp.4000227.
Hagberg H, Gilland E, Bona E, Hanson LA, Hahin-Zoric M, Blennow M, et al. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatr Res 1996;40(4):603-9.
https://doi.org/10.1203/00006450-199610000-00015.
Szaflarski J, Burtrum D, Silverstein FS. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke 1995;26(6):1093-100.
https://doi.org/10.1161/01.STR.26.6.1093.
Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana G, et al. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res 2003;53(4):600-7. https://doi.org/10.1203/01.PDR.0000056802.22454.AB.
Miller LC, Isa S, LoPreste G, Schaller JG, Dinarello CA. Neonatal interleukin-1β, interleukin-6 and tumor necrosis factor: cord blood levels and cellular production. J Pediatr 1990;117(6):961-5. https://doi.org/10.1016/S0022-3476(05)80145-3.
Dammann O, O'Shea TM. Cytokines and perinatal brain damage. Clin Perinatol 2008; 35(4):643-63. https://doi.org/10.1016/j.clp.2008.07.011.
Megyeri P, Abrahám CS, Temesvári P, Kovács J, Vas T, Speer CP. Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood-brain barrier permeability in newborn piglets. Neurosci Lett 1992;148(1-2):137-40.
https://doi.org/10.1016/0304-3940(92)90823-P.
Martin-Ancel A, García-Alix A, Pascual-Salcedo D, Cabañas F, Valcarce M, Quero J. Interleukin-6 in the cerebrospinal fluid after perinatal asphyxia is related to early and late neurological manifestations. Pediatrics 1997;100(5):789-94.
https://doi.org/10.1542/peds.100.5.789.
Oygur N, Sonmez O, Saka O, Yegin O. Predictive value of plasma and cerebrospinal fluid tumor necrosis factor-alpha and interleukin-1 beta concentrations on outcome of full term infants with hypoxic-ischemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 1998;79(3):F190-3. https://doi.org/10.1136/fn.79.3.F190.
Shalak LF, Laptook AR, Jafri HS, Ramilo O, Perlman JM. Clinical chorioamnionitis, elevated cytokines and brain injury in term infants. Pediatrics 2002;110(4):673-80.
https://doi.org/10.1542/peds.110.4.673.
Silveira RC, Procianoy RS. Interleukin-6 and tumor necrosis factor-alpha levels in plasma and cerebrospinal fluid of term newborn infants with hypoxic-ischemic encephalopathy. J Pediatr 2003;143(5):625-9. https://doi.org/10.1067/S0022-3476(03)00531-6.
Aly H, Khashaba MT, El-Ayouty M, El-Sayed O, Hasanein BM. IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev 2006;28(3):178-82. https://doi.org/10.1016/j.braindev.2005.06.006.
Hedtjärn M, Mallard C, Arvidsson P, Hagberg H. White matter injury in the immature brain: role of interleukin-18. Neurosci Lett 2005;373(1):16-20. http://dx.doi.org/10.1016/j.neulet.2004.09.062.
Hedtjärn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci 2002;22(14):5910-9. https://dx.doi.org/20026587.
American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Relationship between perinatal factors and neurologic outcome. In: Poland RL, Freeman RK, editors. Guidelines for perinatal care. 3rd ed. USA, IL: Elk Grove Village AAP; 1992. p. 221-4.
Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol 1976;33(10):696-705. DOI:10.1001/archneur.1976.00500100030012.
Amiel-Tison C, Ellison P. Birth asphyxia in the fullterm newborn: early assessment and outcome. Dev Med Child Neurol 1986;28(5):671-82.
https://dx.doi.org/10.1111/j.1469-8749.1986.tb03914.x.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92(4):529-34. https://doi.org/10.1016/S0022-3476(78)80282-0.
Good PI. Analyzing the large number of variables in biomedical and satellite imagery. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2011. https://doi.org/10.1002/9780470937273.ch2.
Farber JL, Chien KR, Mittnacht S Jr. Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am J Pathol 1981;102(2):271-81.
Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res 1997;41(5):599-606. https://doi.org/10.1203/00006450-199705000-00001.
Johnston MV, Trescher WH, Ishida A, Nakajima W, Zipursky A. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res 2001;49(6):735-41. https://doi.org/10.1203/00006450-200106000-00003.
Vanucci RC. Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res 1990;27(4 Pt 1):317-26.
https://doi.org/10.1203/00006450-199004000-00001.
Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2004;89(2):F152-5. https://dx.doi.org/10.1136/adc.2002.023093.
Clark WM. Cytokines and reperfusion injury. Neurology 1997;49(5 Suppl 4):S10-4.
https://doi.org/10.1212/WNL.49.5_Suppl_4.S10.
Cohen MC, Cohen S. Cytokine function: a study in biologic diversity. Am J Clin Pathol 1996;105(5):589-98. https://doi.org/10.1093/ajcp/105.5.589.
Hill A, Volpe JJ. Pathogenesis and management of hypoxic-ischemic encephalopathy in the term newborn. Neurol Clin 1985;3(1):31-46.
Dammann D, Leviton A. Brain damage in preterm newborns: biological response modification as a strategy to reduce disabilities. J Pediatr 2000;136(4):433-8.
https://doi.org/10.1016/S0022-3476(00)90004-0.
Sävman K, Blennow M, Gustafson K, Tarkowski E, Hagberg H. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr Res 1998;43(6):746-51. https://dx.doi.org/10.1203/00006450-199806000-00006.
Boskabadi H, Afshari JT, Ghayour-Mobarhan M, Maamouri G, Shakeri MT, Sahebkar A et al. Association between serum interleukin-6 levels and severity of perinatal asphyxia. Asian Biomed 2010;4(1):79-85.
Fotopoulos S, Pavlou K, Skouteli H, Papassotiriou I, Lipsou N, Xanthou M. Early markers of brain damage in premature low-birth-weight neonates who suffered from perinatal asphyxia and/or infection. Biol Neonate 2001;79(3-4):213-8. https://doi.org/10.1159/000047094.
Tarkowski E, Rosengren L, Blomstrand C, Wikkelsö C, Jensen C, Ekholm S. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke 1995; 26(8):1393-8. https://doi.org/10.1161/01.STR.26.8.1393.
Jenkins DD, Rollins LG, Perkel JK, Wagner CL, Katikaneni LP, Bass WT, et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Cerebr Blood Flow Metab 2012;32(10):1888-96. https://doi.org/10.1038/jcbfm.2012.83.
Silverstein FS, Barks JD, Hagan P, Liu XH, Ivacko J, Szaflarski J. Cytokines and perinatal brain injury. Neurochem Int 1997;30(4-5):375-83.
https://doi.org/10.1016/S0197-0186(96)00072-1.
Herx LM, Rivest S, Yong VW. Central nervous system initiated inflammation and neurotrophism in trauma IL-1β is required for the production of ciliary neurotrophic factor. J Immunol 2000;165(4):2232-9. https://doi.org/10.4049/jimmunol.165.4.2232.
Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF-α promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 2001;4(11):1116-22. https://doi.org/10.1038/nn738.
Foster-Barber A, Dickens B, Ferriero DM. Human perinatal asphyxia: correlation of neonatal cytokines with MRI and outcome. Dev Neurosci 2001;23(3):213-8.
https://doi.org/10.1159/000046146.
Yamasaki Y, Shozuhara H, Onodera H, Kogure K. Blocking of interleukin-1 activity is beneficial approach to ischemia brain edema formation. Acta Neurochir Suppl (Wien) 1994;60:300-2. https://doi.org/10.1007/978-3-7091-9334-1_80.
Gubits RM, Burke RE, Casey-McIntosh G, Bandele A, Munell F. Immediate early gene induction after neonatal hypoxia-ischemia. Brain Res Mol Brain Res 1993;18(3):228-38.
https://doi.org/10.1016/0169-328X(93)90194-T.
Felderhoff –Mueser U, Schmidt OI, Oberholzer A, Bührer C, Stahel PF. IL-18: a key player in neuroinflammation and neurodegeneration? Trends Neurosci 2005;28(9):487-93. https://dx.doi.org/10.1016/j.tins.2005.06.008.
Minagawa K, Tsuji Y, Ueda H, Koyama K, Tanizawa K, Okamura H, et al. Possible correlation between high levels of IL-18 in the cord blood of pre-term infants and neonatal development of periventricular leukomalacia and cerebral palsy. Cytokine 2002;17(3):164-70. https://doi.org/10.1006/cyto.2001.0988.
Schmitz T, Heep A, Groenendaal F, Hüseman D, Kie S, Bartmann P, et al. Interleukin-1β, interleukin-18, and interferon-γ expression in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus - markers of white matter damage? Pediatr Res 2007;61(6):722-6. http://dx.doi.org/10.1203/pdr.0b013e31805341f1.
Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 2011;8(1):3.
https://doi.org/10.1186/2045-8118-8-3.
Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 2009;31(4):497-511.
https://doi.org/10.1007/s00281-009-0177-0.
Chen X, ThrelkedSW, Cummings EE, Juan I, Makeyev O, Besio WG, et al. Ischemia-reperfusion impairs blood-brain barrier function and alters tight junction protein expression in the ovine fetus. Neuroscience 2012;226:89-100.https://doi.org/10.1016/j.neuroscience.2012.08.043.
Ellison VJ, Mocatta TJ, Winterbourn CC, Darlow BA, Volpe JJ, Inder TE. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res 2005;57(2):282-6. https://doi.org/10.1203/01.PDR.0000148286.53572.95.
Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des 2005;11(8):973-84. https://doi.org/10.2174/1381612053381684.
Downloads
Additional Files
Published
How to Cite
Accepted 2016-11-15
Published 2017-08-20









