Sinotomy technique versus surgical excision with primary closure technique in pilonidal sinus disease
DOI:
https://doi.org/10.17305/bjbms.2014.139Keywords:
pilonidal sinus, excision, quality of life, recurrence, sinotomyAbstract
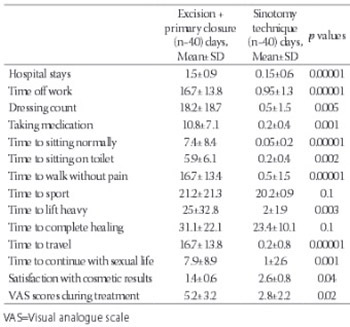
Pilonidal disease is a common chronic disorder mainly seen in the sacrococcygeal region, especially in young males. Many surgical treatment modalities have been suggested, but an ideal and widely accepted treatment has yet to be established. The aim of this study was to compare quality of life of patients treated with the sinotomy technique with quality of life of patients treated with surgical excision plus primary closure technique by means of quality of life questionnaire. The data of patients who had been treated for pilonidal sinus in our clinic from September 2010 to June 2012 were analyzed retrospectively. Forty patients were treated with sinotomy technique and 40 patients were treated with surgical excision plus primary closure technique. Time to return to work and to time to complete wound healing were evaluated. All patients were asked to fill the questionnaire after complete healing occurred. Postoperative complications were bleeding in 2.5%, infection in 3.75% and fever in 2.5% patients. There were no significant differences between the two groups in terms of complete healing (p=0.1) and sport times (p=0.1). There were significant differences between the groups in terms of length of hospital stay (p≤0.001), time off work (p≤0.001),times to sitting on toilet and walking without pain (p=0.002 and p≤0.001,respectively). The mean postoperative VAS scores were 5.2±3.2 and 2.8±2.2, respectively (p=0.02). The technique of sinotomy with good wound and surrounding skin care seems to be an ideal approach with high chance of cure. The patients returned to their routine in a short period of time.
Citations
Downloads
References
Allen-Mersh TG. Pilonidal-sinus: finding the right track for treatment. Br J Surg1990;77(2):123–32. doi:10.1002/bjs.1800770203.
http://dx.doi.org/10.1002/bjs.1800770203
Bendewald FP, Cima RR. Pilonidal disease. Clin Colon Rectal Surg 2007;20(2):86-95.
http://dx.doi.org/10.1055/s-2007-977486
Akin M, Leventoglu S, Mentes BB, Bostanci H, Gokbayir H, Kilic K, et al. Comparison of the classic Limberg flap and modified Limberg flap in the treatment of pilonidal sinus disease: a retrospective analysis of 416 patients. Surg Today 2010;40(8): 757-62. Epub 2010 Jul 30.
http://dx.doi.org/10.1007/s00595-008-4098-7
Milito G, Cortese F, Casciani CU. Rhomboid flap procedure for pilonidal sinus: results from 67 cases. Int J Colorectal Dis 1998; 13(3): 113–5.
http://dx.doi.org/10.1007/s003840050146
Urhan MK, Kucukel F, Topgul K, Ozer I, Sari S. Rhomboid excision and Limberg flap for managing pilonidal sinus: results of 102 cases. Dis Colon Rectum 2002; 45(5): 656–9. doi:10.1007/s10350-004-6263-4.
http://dx.doi.org/10.1007/s10350-004-6263-4
Al-Mulhim AS, Sultan MA, Ahmed HH. Pilonidal Sinus in Males: To preserve or obliterate the natal cleft. Saudi Med Journal 2002;23(7):875-6.
Hull TL, Wu J. Pilonidal disease. Surg Clin North Am 2002;82:1169-1185.
http://dx.doi.org/10.1016/S0039-6109(02)00062-2
Allen-Mersh TG. Pilonidal sinus: finding the right track for treatment. Br J Surg 1990;77:123-132.
http://dx.doi.org/10.1002/bjs.1800770203
Aygen E, Arslan K, Dogru O, Basbug M, Camci C. Crystallized phenol in nonoperative treatment of previously operated, recurrent pilonidal disease. Dis Colon Rectum 2010;53(6):932–5.
http://dx.doi.org/10.1007/DCR.0b013e3181d8283b
Spyridakis M, Christodoulidis G, Chatszitheofilou C, Symeonidis D, Tepetes C. The role of the platelet-rich plasma in accelerating the wound healing process and recovery in patients being operated for pilonidal sinus disease: preliminary results.World J Surg 2009;33(8):1764–1769.
http://dx.doi.org/10.1007/s00268-009-0046-y
Daphan C, Tekelioglu MH, Sayilgan C. Limberg flap repair for pilonidal sinus disease. Dis Colon Rectum. 2004;47(2):233-7.
http://dx.doi.org/10.1007/s10350-003-0037-2
Tocchi A, Mazzoni G, Bononi M, Fornasari V, Miccini M, Drumo A, et al. Outcome of chronic pilonidal disease treatment after ambulatory plain midline excision and primary suture. Am J Surg 2008;196(1):28-33. Comment in: Am J Surg 2009; 197(5):693-4.
http://dx.doi.org/10.1016/j.amjsurg.2007.05.051
Goligher J, Duthie H, Nixon H. Pilonidal sinus. In: Tindall B, ed. Surgery of the anus rectum and colon. 5th. London: 1992:230-233.
Irkörücü O, Erdem H, Reyhan E. The best therapy for pilonidal disease: which management for which type? World J Surg 2012;36:691-692.
http://dx.doi.org/10.1007/s00268-011-1285-2
da Silva JH. Pilonidal cyst: cause and treatment. Dis Colon Rectum 2000; 43(8): 1146-56.
http://dx.doi.org/10.1007/BF02236564
Bozkurt MK, Tezel E. Management of pilonidal sinus with the Limberg flap. Dis Colon Rectum 1998;41(6):775–777.
http://dx.doi.org/10.1007/BF02236268
Kapan M, Kapan S, Pekmezci S, Durgun V. Sacrococcygeal pilonidal sinus disease with Limberg flap repair. Tech Coloproctol 2002;6(1):27-32. doi:10.1007/s101510200005
http://dx.doi.org/10.1007/s101510200005
Ozgultekin R, Ersan Y, Ozcan M. Die therapie des sinus pilonidalis mit dem transpositionslappen nach Limberg. Chirurg 1995;66(3):192–195.
Bascom J, Bascom B. Failed pilonidal surgery: new paradigm and new operation leading to cures. Arch Surg 2002;137(10):1146–50. http://dx.doi.org/10.1001/archsurg.137.10.1146
Iesalnieks I, Furst A, Rentsch M, Jauch KW. Primary midline closure after excision of a pilonidal sinus is associated with a high recurrence rate. Chirurg 2003;74(5):461–8. doi:10.1007/s00104-003-0616-8.
http://dx.doi.org/10.1007/s00104-003-0616-8
Rakinic J. Sacrococcygeal pilonidal sinus. In:Cameron JL, ed. Current surgical therapy. 8th. St. Louis, Missouri: Mosby, 1998: 302-306.
Lee HC, Ho YH, Seow CF, Eu KW, Nyam D. Pilonidal disease in Singapore: clinical features and management. Aust N Z J Surg 2000;70(3):196-198.
http://dx.doi.org/10.1046/j.1440-1622.2000.01785.x
Zieger K. Complications after surgery for pilonidal cyst. An introduction to a new debate on a “costly” disease. Ugeskr Laeger 1999;161(44):6056 – 58.
Ertan T, Koc M, Gocmen E, Aslar AK, Keskek M, Kilic M. Does technique alter quality of life after pilonidal sinus surgery? Am J Surg 2005;190(3):388-92.
http://dx.doi.org/10.1016/j.amjsurg.2004.08.068
Abu Galala KH, Salam IM, Abu Samaan KR, El Ashaal YI, Chandran VP, Sabastian M, et al. Treatment of pilonidal sinus by primary closure with a transposed rhomboid flap compared with deep suturing: a prospective randomized clinical trial. Eur J Surg1999;165(5):468-72.
http://dx.doi.org/10.1080/110241599750006721
Miocinovic M, Horzic M, Bunoza D. The treatment of pilonidal disease of the sacrococcygeal region by the method of limited excision and open wound healing. Acta Med Croatica 2000:54(1): 27-31.
Al-Naami MY. Outpatient pilonidal sinotomy complemented with good wound and surrounding skin care. Saudi Med J 2005;26(2):285–8.
Yalcin S, Ergul E. A single-surgeon, single-institute experience of 59 sinotomies for sacrococcygeal pilonidal disease under local anesthesia. Bratisl Lek Listy 2010;111(5):284-5.
Rabie ME, Al Refeidi AA, Al Haizaee A, Hilal S, Al Ajmi H, Al Amri AA. Sacrococcygeal pilonidal disease: sinotomy versus excisional surgery, a retrospective study. ANZ J Surg. 2007;77(3):177-80.
http://dx.doi.org/10.1111/j.1445-2197.2006.04002.x
Aydede H, Erhan Y, Sakarya A, Kumkumoglu Y. Comparison of three methods in surgical treatment of pilonidal disease. ANZ J Surg 2001;71(6):362–364. http://dx.doi.org/10.1046/j.1440-1622.2001.02129.x
Kayaalp C, Aydin C. Review of phenol treatment in sacrococcygeal pilonidal disease. Tech Coloproctol 2009;13(3):189–193.
http://dx.doi.org/10.1007/s10151-009-0519-x
Lee SL, Tejirian T, Abbas MA. Current management of adolescent pilonidal disease. J Pediatr Surg 2008;43(6):1124–1127. http://dx.doi.org/10.1016/j.jpedsurg.2008.02.0422.042.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2015 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2014-10-19
Published 2014-11-12









