Unknown primary adenocarcinomas: a single-center experience
DOI:
https://doi.org/10.17305/bjbms.2016.1495Keywords:
adenocarcinoma, neoplasm metastasis, prognosisAbstract
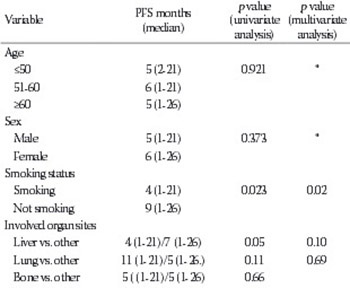
This study aimed to elucidate the clinical and prognostic characteristics of a homogeneous group of patients with cancer of unknown primary (CUP). Between 1999 and 2014, CUP was diagnosed in 159 (1.3%) of 11,742 cancer patients at Trakya University Hospital (Edirne, Turkey). Ninety-seven (61%) of the 159 patients were retrospectively reviewed. Among these, 61 (62.8%) patients with adenocarcinoma were included in this study. The most frequently predicted primary tumor site was the lung (37.7%), and 59% of the patients were smokers. There was a significant relationship between smoking and the lung as a potential primary cancer site (p = 0.042). The most frequent site of metastasis was the liver (60.7%). The median number of metastases per patient was two, but patients with liver metastases had a median of five metastases. The overall median survival time was 7 months. Median survival was significantly longer in patients with a predicted primary site than in patients without the predicted site (7 vs. 6 months, respectively; p = 0.038). When the patients with predicted ovarian and peritoneal tumors were excluded from the comparison, the statistical p value was still close to significant (p = 0.07). Multivariate analysis revealed that smoking, liver metastasis, serum alkaline phosphatase ≥92 U/L, and progression in response to chemotherapy were independent predictors of a poor prognosis. The present study identified several independent prognostic factors in patients with unknown primary adenocarcinomas who received chemotherapy. Smoking, the presence of liver metastasis, and response to chemotherapy were independent risk factors for both progression-free and overall survival.
Citations
Downloads
References
Abbruzzese JL, Abbruzzese MC, Hess KR, Raber MN, Lenzi R, Frost P. Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol. 1994;12(6):1272-80.
Pavlidis N, Pentheroudakis G. Cancer of unknown primary site: 20 questions to be answered. Ann Oncol. 2010;21(Suppl 7):vii303-7. DOI: 10.1093/annonc/mdq278.
Pavlidis N, Fizazi K. Cancer of unknown primary (CUP). Crit Rev Oncol Hematol. 2005;54(3):243-50. http://dx.doi.org/10.1016/j.critrevonc.2004.10.002.
van de Wouw AJ, Janssen-Heijnen ML, Coebergh JW, Hillen HF. Epidemiology of unknown primary tumours; incidence and population-based survival of 1285 patients in Southeast Netherlands, 1984-1992. Eur J Cancer. 2002;38(3):409-13.
http://dx.doi.org/10.1016/S0959-8049(01)00378-1.
Altman E, Cadman E. An analysis of 1539 patients with cancer of unknown primary site. Cancer. 1986;57(1):120-4.
http://dx.doi.org/10.1002/1097-0142(19860101)57:1<120::AID-CNCR2820570124>3.0.CO;2-M.
Pentheroudakis G, Briasoulis E, Pavlidis N. Cancer of unknown primary site: missing primary or missing biology? Oncologist. 2007;12(4):418-25.
http://dx.doi.org/10.1634/theoncologist.12-4-418.
van de Wouw AJ, Jansen RL, Speel EJ, Hillen HF. The unknown biology of the unknown primary tumour: a literature review. Ann Oncol. 2003;14(2):191-6.
http://dx.doi.org/10.1093/annonc/mdg068.
Seve P, Sawyer M, Hanson J, Broussolle C, Dumontet C, Mackey JR. The influence of comorbidities, age, and performance status on the prognosis and treatment of patients with metastatic carcinomas of unknown primary site: a population-based study. Cancer. 2006;106(9):2058-66. http://dx.doi.org/10.1002/cncr.21833.
Lazaridis G, Pentheroudakis G, Fountzilas G, Pavlidis N. Liver metastases from cancer of unknown primary (CUPL): a retrospective analysis of presentation, management and prognosis in 49 patients and systematic review of the literature. Cancer Treat Rev. 2008;34(8):693-700. http://dx.doi.org/10.1016/j.ctrv.2008.05.005.
Seve P, Ray-Coquard I, Trillet-Lenoir V, Sawyer M, Hanson J, Broussolle C, et al. Low serum albumin levels and liver metastasis are powerful prognostic markers for survival in patients with carcinomas of unknown primary site. Cancer. 2006;107(11):2698-705.
http://dx.doi.org/10.1002/cncr.22300.
Shaw PH, Adams R, Jordan C, Crosby TD. A clinical review of the investigation and management of carcinoma of unknown primary in a single cancer network. Clin Oncol (R Coll Radiol). 2007;19(1):87-95. http://dx.doi.org/10.1016/j.clon.2006.09.009.
van de Wouw AJ, Jansen RL, Griffioen AW, Hillen HF. Clinical and immunohistochemical analysis of patients with unknown primary tumour. A search for prognostic factors in UPT. Anticancer Res. 2004;24(1):297-301.
Thom I, Rogers C, Andritzky B, Witzel I, Schuch G, Hossfeld DK, et al. Single-center management of 136 patients with cancer of unknown primary site (CUP syndrome) over a period of 10 years. Onkologie. 2009;32(12):741-6.
http://dx.doi.org/10.1159/000252797.
Hainsworth JD, Johnson DH, Greco FA. Cisplatin-based combination chemotherapy in the treatment of poorly differentiated carcinoma and poorly differentiated adenocarcinoma of unknown primary site: results of a 12-year experience. J Clin Oncol. 1992;10(6):912-22.
Trivanovic D, Petkovic M, Stimac D. New prognostic index to predict survival in patients with cancer of unknown primary site with unfavourable prognosis. Clin Oncol (R Coll Radiol). 2009;21(1):43-8. http://dx.doi.org/10.1016/j.clon.2008.09.007.
Culine S, Kramar A, Saghatchian M, Bugat R, Lesimple T, Lortholary A, et al. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol. 2002;20(24):4679-83.
http://dx.doi.org/10.1200/JCO.2002.04.019.
Chen KW, Liu CJ, Lu HJ, Tzeng CH, Liu JH, Chiou TJ, et al. Evaluation of prognostic factors and the role of chemotherapy in unfavourable carcinoma of unknown primary site: a 10-year cohort study. BMC Research Notes. 2012;5:70.
http://dx.doi.org/10.1186/1756-0500-5-70.
Pasterz R, Savaraj N, Burgess M. Prognostic factors in metastatic carcinoma of unknown primary. J Clin Oncol. 1986;4(11):1652-7.
Stewart JF, Tattersall MH, Woods RL, Fox RM. Unknown primary adenocarcinoma: incidence of overinvestigation and natural history. Br Med J. 1979;1(6177):1530-3. http://dx.doi.org/10.1136/bmj.1.6177.1530.
van der Gaast A, Verweij J, Planting AS, Hop WC, Stoter G. Simple prognostic model to predict survival in patients with undifferentiated carcinoma of unknown primary site. J Clin Oncol. 1995;13(7):1720-5.
Greco FA, Erland JB, Morrissey LH, Burris HA 3rd, Hermann RC, Steis R, et al. Carcinoma of unknown primary site: phase II trials with docetaxel plus cisplatin or carboplatin. Ann Oncol. 2000;11(2):211-5. http://dx.doi.org/10.1023/A:1008369812295.
Kramer A, Hubner G, Schneeweiss A, Folprecht G, Neben K. Carcinoma of unknown primary - an orphan disease? Breast Care (Basel). 2008;3(3):164-70. DOI: 10.1159/000136001.
Pavlidis N. Optimal therapeutic management of patients with distinct clinicopathological cancer of unknown primary subsets. Ann Oncol. 2012;23(Suppl 10):x282-5.
DOI: 10.1093/annonc/mds317.
Gatalica Z, Millis SZ, Vranic S, Bender R, Basu GD, Voss A, et al. Comprehensive tumor profiling identifies numerous biomarkers of drug response in cancers of unknown primary site: analysis of 1806 cases. Oncotarget. 2014;5(23):12440-7.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-07-13
Published 2016-11-10









