The efficacy of modified docetaxel-cisplatin-5-fluorouracil regimen as first-line treatment in patients with alpha-fetoprotein producing gastric carcinoma
DOI:
https://doi.org/10.17305/bjbms.2017.1684Keywords:
Alpha-fetoprotein, alpha-fetoprotein producing gastric carcinoma, gastric carcinoma, modified docetaxel-cisplatin-5-fluorouracil, chemotherapyAbstract
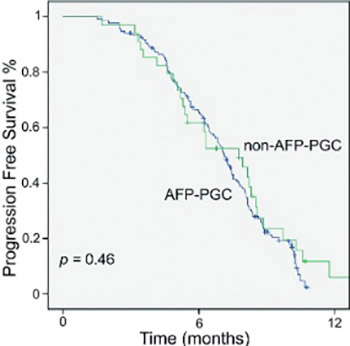
Alpha-fetoprotein producing gastric carcinoma (AFP-PGC) is a rare cancer for which limited data on the clinicopathological features and treatment modalities exist. The aim of this study was to compare the efficacy of modified docetaxel-cisplatin-5-fluorouracil (mDCF) as the first-line chemotherapy regimen in metastatic AFP-PGC and non-AFP-PGC. The patients diagnosed with metastatic gastric cancer who were given mDCF as first-line therapy were retrospectively reviewed. The patients with a basal serum AFP level over 9 ng/ml were defined as AFP-PGC patients. In total, 169 patients (34 with AFP-PGC and 135 with non-AFP-PGC) were included in this study. AFP-PGC patients had more liver metastases than non-AFP-PGC patients (p < 0.001). A decrease in basal AFP levels after three cycles of chemotherapy was significantly different in AFP-PGC group (p = 0.001).Overall disease control rate was 79.4% (partial response [PR] - 44.1%, stable disease [SD] - 35.3%), and 82.2% (complete response - 3%, PR - 36.2%, SD - 43%) in AFP-PGC and non-AFP-PGC patients, respectively. There was no difference between AFP-PGC and non-AFP-PGC groups in overall and progression-free survival rates (11.3 versus 11.4 months and 7.7 versus 7.1 months, respectively). Rates of grade 3-4 hematologic toxicity were 8.8% and 6.7% for neutropenia in AFP-PGC and non-AFP-PGC group, respectively and 5.9% and 7.4% for anemia. In conclusion, mDCF regimen is well-tolerated with acceptable toxicity outcomes in both AFP-PGC and non-AFP-PGC patients. A statistically significant decrease in AFP levels after mDCF regimen indicate that AFP might be considered as a supplemental marker of response to mDCF chemotherapy in AFP-PGC patients. However, further prospective clinical trials are required in this area.
Citations
Downloads
References
Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest 1956;8(2):174. https://doi.org/10.3109/00365515609049266.
El-Bahrawy M. Alpha-fetoprotein-producing non-germ cell tumours of the female genital tract. Eur J Cancer 2010;46(8):1317-22. https://doi.org/10.1016/j.ejca.2010.01.028.
Liu X, Cheng Y, Sheng W, Lu H, Xu X, Xu Y, et al. Analysis of clinicopathologic features and prognostic factors in hepatoid adenocarcinoma of the stomach. Am J Surg Pathol 2010;34(10):1465-71. https://doi.org/10.1097/PAS.0b013e3181f0a873.
Sterling RK, Wright EC, Morgan TR, Seeff LB, Hoefs JC, Di Bisceglie AM, et al. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol 2012;107(1):64-74. https://doi.org/10.1038/ajg.2011.312.
Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology 1998;27(1):273-8. https://doi.org/10.1002/hep.510270140.
Hamanaka W, Yoneda S, Shirakusa T, Shirahama H, Tashiro Y, Iwasaki A, et al. Alpha-fetoprotein (AFP)-producing adrenocortical carcinoma – Long survival with various therapeutic strategies including a lung resection: Report of a case. Surg Today 2008;38(3):275-8. https://doi.org/10.1007/s00595-007-3610-9.
Saito S, Hatano T, Hayakawa M, Koyama Y, Ohsawa A, Iwamasa T. Studies on alpha-fetoprotein produced by renal cell carcinoma. Cancer 1989;63(3):544-9. https://doi.org/10.1002/1097-0142(19890201)63:3<544::AID-CNCR2820630324>3.0.CO;2-2.
Sun N, Sun Q, Liu Q, Zhang T, Zhu Q, Wang W, et al. α-fetoprotein-producing gastric carcinoma: A case report of a rare subtype and literature review. Oncol Lett 2016;11(5):3101-4. https://doi.org/10.3892/ol.2016.4372.
Li XD, Wu CP, Ji M, Wu J, Lu B, Shi HB, et al. Characteristic analysis of α-fetoprotein-producing gastric carcinoma in China. World J Surg Oncol 2013;11(1):246. https://doi.org/10.1186/1477-7819-11-246.
Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, et al. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg 2002;19(5):359-65. https://doi.org/10.1159/000065838.
Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: Multivariate analysis of prognostic factors in 270 patients. Oncology 2003;65(2):95-101. https://doi.org/ DOI:10.1159/000072332.
Koide N, Nishio A, Igarashi J, Kajikawa S, Adachi W, Amano J. Alpha-fetoprotein-producing gastric cancer: Histochemical analysis of cell proliferation, apoptosis, and angiogenesis. Am J Gastroenterol 1999;94(6):1658-63. https://doi.org/10.1111/j.1572-0241.1999.01158.x; https://doi.org/10.1016/S0002-9270(99)00214-2.
Sun W, Liu Y, Shou D, Sun Q, Shi J, Chen L, et al. AFP (alpha fetoprotein): who are you in gastrology? Cancer Lett 2015;357(1):43-6. https://doi.org/10.1016/j.canlet.2014.11.018.
Keskin S, Yildiz I, Sen F, Aydogan F, Kilic L, Ekenel M, et al. Modified DCF (mDCF) regimen seems to be as effective as original DCF in advanced gastric cancer (AGC). Clin Transl Oncol 2013;15(5):403-8. https://doi.org/10.1007/s12094-012-0942-8.
Ozdemir NY, Abali H, Oksüzoğlu B, Budakoglu B, Uncu D, Guler T, et al. The efficacy and safety of reduced-dose docetaxel, cisplatin, and 5-fluorouracil in the first-line treatment of advanced stage gastric adenocarcinoma. Med Oncol 2010;27(3):680-4. https://doi.org/10.1007/s12032-009-9268-y.
Chi Y, Ren JH, Yang L, Cui CX, Li JL, Wang JW. Phase II clinical study on the modified DCF regimen for treatment of advanced gastric carcinoma. Chin Med J (Engl) 2011;124(19):2997-3002.
McIntire KR, Waldmann TA, Moertel CG, Go VL. Serum alpha-fetoprotein in patients with neoplasms of the gastrointestinal tract. Cancer Res 1975;35(4):991-6.
Inoue M, Sano T, Kuchiba A, Taniguchi H, Fukagawa T, Katai H. Long-term results of gastrectomy for alpha-fetoprotein-producing gastric cancer. Br J Surg 2010;97(7):1056-61. https://doi.org/10.1002/bjs.7081.
Chun H, Kwon SJ. Clinicopathological characteristics of alpha-fetoprotein-producing gastric cancer. J Gastric Cancer 2011;11(1):23-30. https://doi.org/10.5230/jgc.2011.11.1.23.
Horie Y, Miura K, Matsui K, Yukimasa A, Ohi S, Hamamoto T, et al. Marked elevation of plasma carcinoembryonic antigen and stomach carcinoma. Cancer 1996;77(4):1991-7. https://doi.org/10.1002/(SICI)1097-0142(19960515)77:10<1991::AID-CNCR5>3.0.CO;2-K.
Carpelan-Holmström M, Louhimo J, Stenman UH, Alfthan H, Haglund C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res 2002;22(4):2311-6.
Zhang JF, Shi SS, Shao YF, Zhang HZ. Clinicopathological and prognostic features of hepatoid adenocarcinoma of the stomach. Chin Med J (Engl) 2011;124(10):1470-6.
Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther 2008;25(10):1075-84. https://doi.org/10.1007/s12325-008-0100-4.
Liu X, Yang M, Gao J, Zhang S, Xi Y. Clinicopathologic features and prognosis of 51 patients with α-fetoprotein-producing gastric cancer. [Article in Chinese]. Zhonghua Zhong Liu Za Zhi 2015;37(3):231-4.
Chang YC, Nagasue N, Kohno H, Tanjura H, Uchida M, Yamanoi A, et al. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol 1990;85(11):1480-5.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Corrado B, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J Clin Oncol 2006;24(31):4991-7. https://doi.org/10.1200/JCO.2006.06.8429.
Kos FT, Uncu D, Ozdemir N, Budakoglu B, Odabas H, Abali H, et al. Comparison of cisplatin-5-fluorouracil- folinic acid versus modified docetaxel-cisplatin-5-fluorouracil regimens in the first-line treatment of metastatic gastric cancer. Chemotherapy 2011;57(3):230-5. https://doi.org/10.1159/000327840.
Inal A, Kaplan MA, Kucukoner M, Isikdogan A. Docetaxel and cisplatin plus fluorouracil compared with modified docetaxel, cisplatin, and 5-fluorouracil as first-line therapy for advanced gastric cancer: A retrospective analysis of single institution. Neoplasma 2012;59(2):233-6.https://doi.org/10.4149/neo_2012_030.
Amemiya H, Kono K, Mori Y, Takahashi A, Ichihara F, Iizuka H, et al. High frequency of c-MET expression in gastric cancers producing a-fetoprotein. Oncology 2009;59(2):145-51. https://doi.org/10.1159/000012152.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-11-15
Published 2017-05-20









