The role of narrow band imaging in colorectal polyp detection
DOI:
https://doi.org/10.17305/bjbms.2017.1686Keywords:
Colonoscopy, narrow band imaging, polyp detection rate, adenoma detection rate, colorectal cancerAbstract
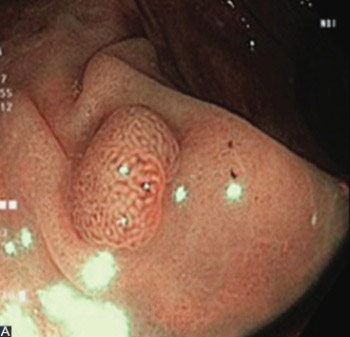
Colonoscopic detection and removal of polyps represent the most important prevention method for colorectal cancer. We aimed to investigate the diagnostic yield of narrow band imaging (NBI) colonoscopy for polyp detection compared with standard colonoscopy. In this prospective study, 505 patients that underwent total colonoscopy were randomized into two groups: 226 patients in NBI group and 279 in non-NBI group (standard colonoscopy). The primary endpoints were polyp detection rate (PDR) and adenoma detection rate (ADR) in both groups. Polyps detected with NBI technique were characterized according to the NBI International Colorectal Endoscopic (NICE) classification. The total number of polyps detected in NBI group was significantly higher compared with non-NBI group (325 polyps in 226 patients versus 189 polyps in 279 patients, respectively). PDR in NBI group was 55.3%, versus 43.3% in non-NBI group. ADR in NBI group was significantly higher compared with non-NBI group (35.3% versus 20%, respectively). The proportion of detected adenomas in the left-sided colon was significantly higher in NBI group (72.8% versus 61.06% in non-NBI group), which was related to an increased number of small adenomas detected in NBI group. Also, in NBI group, a significant number of flat adenomas were detected (28 versus 9 in non-NBI group). A total of 147 (45.2%) polyps were classified according to the NICE classification, and showed a good correlation with histological analysis. In conclusion, this study demonstrated increased PDR and ADR for NBI colonoscopy. A good correlation between the NICE classification and histological analysis was also observed.
Citations
Downloads
References
Winawer SJ, Zauber AG. Colonoscopic polypectomy and the incidence of colorectal cancer. Gut 2001;48(6):753-4. https://doi.org/10.1136/gut.48.6.753.
Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329(27):1977-81. https://doi.org/10.1056/NEJM199312303292701.
Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, et al. Colonoscopic miss rate of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112(1):24-8.
https://doi.org/10.1016/S0016-5085(97)70214-2.
Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol 2006;101(12):2866-77.
https://doi.org/10.1111/j.1572-0241.2006.00905.x.
Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, et al. Miss rate for colorectal neoplastic polyps: A prospective multicenter study of back-to-back video colonoscopies. Endoscopy 2008;40(4):284-90. https://doi.org/10.1055/s-2007-995618.
Consolo P, Luigiano C, Strangio G, Scaffidi MG, Giacobbe G, Di Giuseppe G, et al. Efficacy, risk factors and complications of endoscopic polypectomy: Ten year experience at a single center. World J Gastroenterol 2008;14(15):2364-9. https://doi.org/10.3748/wjg.14.2364.
Sano Y, Saito Y, Fu KI, Matsuda T, Uraoka T, Kobayashi N, et al. Efficacy of magnifying chromoendoscopy for the differential diagnosis of colorectal lesions. Digestive Endoscopy 2005;17(2):105-16.
https://doi.org/10.1111/j.1443-1661.2005.00483.x.
Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol 2006;101(12):2711-6.
https://doi.org/10.1111/j.1572-0241.2006.00932.x.
Ng SC, Lau JY. Narrow-band imaging in the colon: Limitations and potentials. J Gastroenterol Hepatol 2011;26(11):1589-96.
https://doi.org/10.1111/j.1440-1746.2011.06877.x.
Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt 2004;9(3):568-77. https://doi.org/10.1117/1.1695563.
Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: A prospective controlled study. Endoscopy 2007;39(12):1092-6. https://doi.org/10.1055/s-2007-966781.
Rastogi A, Bansal A, Wani S, Callahan P, McGregor DH, Cherian R, et al. Narrow-band imaging colonoscopy - a pilot feasibility study for the detection of polyps and correlation of surface patterns with polyp histologic diagnosis. Gastrointest Endosc 2008;67(2):280-6. https://doi.org/10.1016/j.gie.2007.07.036.
Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer 2001;84(10):1354-62. https://doi.org/10.1054/bjoc.2001.1809.
Uraoka T, Saito Y, Matsuda T, Sano Y, Ikehara H, Mashimo Y, et al. Detectability of colorectal neoplastic lesions using a narrow-band imaging system: A pilot study. J Gastroenterol Hepatol 2008;23(12):1810-5. https://doi.org/10.1111/j.1440-1746.2008.05635.x.
Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012;143(3):599-607. https://doi.org/10.1053/j.gastro.2012.05.006.
Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47(2):251-5. https://doi.org/10.1136/gut.47.2.251.
Rex DC, Helbig CC. High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology 2007;133(1):42-7. https://doi.org/10.1053/j.gastro.2007.04.029.
Adler A, Aschenbeck J, Yenerim T, Mayr M, Aminalai A, Drossel R, et al. Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: A prospective randomized trial. Gastroenterology 2009;136(2):410-6. https://doi.org/10.1053/j.gastro.2008.10.022.
Dinesen L, Chua TJ, Kaffes AJ. Meta-analysis of narrow-band imaging versus conventional colonoscopy for adenoma detection. Gastrointest Endosc 2012;75(3):604-11. https://doi.org/10.1016/j.gie.2011.10.017.
Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, et al. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: A meta-analysis. Am J Gastroenterol 2012;107(3):363-70. https://doi.org/10.1038/ajg.2011.436.
Inoue T, Murano M, Murano N, Kuramoto T, Kawakami K, Abe Y, et al. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: A randomized, controlled trial. J Gastroenterol 2008;43(1):45-50.
https://doi.org/10.1007/s00535-007-2125-x.
East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: A pilot study. Gastrointest Endosc 2007;66(2):310-6. https://doi.org/10.1016/j.gie.2007.02.026.
Rastogi A, Pondugula K, Bansal A, Wani S, Keighley J, Sugar J, et al. Recognition of surface mucosal and vascular patterns of colon polyps by using narrow-band imaging: Interobserver and intraobserver agreement and prediction of polyps histology. Gastrointest Endosc 2009;69(3 Pt 2):716-22. https://doi.org/10.1016/j.gie.2008.09.058.
Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, et al. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut 2007;56(3):373-9. https://doi.org/10.1136/gut.2006.099614.
Zhou QJ, Yang JM, Fei BY, Xu QS, Wu WQ, Ruan HJ. Narrow-band imaging endoscopy with and without magnification in diagnosis of colorectal neoplasia. World J Gastroenterol 2011;17(5):666-70. https://doi.org/10.3748/wjg.v17.i5.666.
East JE, Suzuki N, Stavrinidis M, Guenther T, Thomas HJ, Saunders BP. Narrow band imaging for colonic surveillance in hereditary non-polyposis colorectal cancer. Gut 2008;57(1):65-70. https://doi.org/10.1136/gut.2007.128926.
Kuiper T, Marsman WA, Jansen JM, van Soest EJ, Haan YC, Bakker GJ, et al. Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol 2012;10(9):1016-20. https://doi.org/10.1016/j.cgh.2012.05.004.
Rex DK, Overhiser AJ, Chen SC, Cummings OW, Ulbright TM. Estimation of impact of American College of Radiology recommendations on CT colonography reporting for resection of high-risk adenoma findings. Am J Gastroenterol 2009;104(1):149-53. https://doi.org/10.1038/ajg.2008.35.
Rex DK, Kahi C, O'Brien M, Levin TR, Pohl H, Rastogi A, et al. The American Society for Gastrointestinal Endoscopy PIVI (Prevention an Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2011;73(3):419-22. https://doi.org/10.1016/j.gie.2011.01.023.
Hewett DG, Huffman ME, Rex DK. Leaving distal colorectal hyperplastic polyps in place can be achieved with high accuracy by using narrow-band imaging: An observational study. Gastrointest Endosc 2012;76(2):374-80. https://doi.org/10.1016/j.gie.2012.04.446.
Paggi S, Rondonotti E, Amato A, Terruzzi V, Imperiali G, Mandelli G, et al. Resect and discard strategy in clinical practice: A prospective cohort study. Endoscopy 2012;44(10):899-904. https://doi.org/10.1055/s-0032-1309891.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-11-17
Published 2017-05-20









