A silent mutation in human alpha-A crystallin gene in patients with age-related nuclear or cortical cataract
DOI:
https://doi.org/10.17305/bjbms.2017.1745Keywords:
Single nucleotide polymorphism, SNP, restriction fragment length polymorphism, crystallin, age-related cataract, nuclear cataract, cortical cataract, crystallin alpha A, crystallin alpha B, CRYAA, CRYAB, silent mutationAbstract
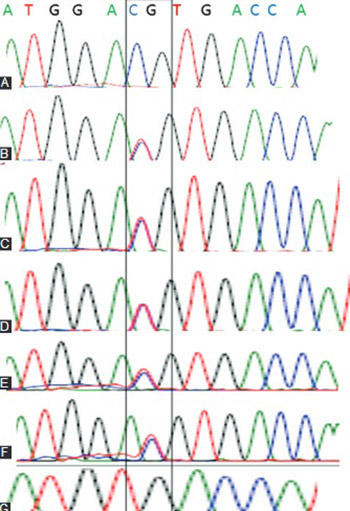
A cataract is a complex multifactorial disease that results from alterations in the cellular architecture, i.e. lens proteins. Genes associated with the development of lens include crystallin genes. Although crystallins are highly conserved proteins among vertebrates, a significant number of polymorphisms exist in human population. In this study, we screened for polymorphisms in crystallin alpha A (CRYAA) and alpha B (CRYAB) genes in 200 patients over 40 years of age, diagnosed with age-related cataract (ARC; nuclear and cortical cataracts). Genomic DNA was extracted from the peripheral blood. The coding regions of the CRYAA and CRYAB gene were amplified using polymerase chain reaction and subjected to restriction digestion. Restriction fragment length polymorphism (RFLP) was performed using known restriction enzymes for CRYAA and CRYAB genes. Denaturing high performance liquid chromatography and direct sequencing were performed to detect sequence variation in CRYAA gene. In silico analysis of secondary CRYAA mRNA structure was performed using CLC RNA Workbench. RFLP analysis did not show any changes in the restriction sites of CRYAA and CRYAB genes. In 6 patients (4 patients with nuclear cataract and 2 with cortical cataract), sequence analysis of the exon 1 in the CRYAA gene showed a silent single nucleotide polymorphism [D2D] (CRYAA: C to T transition). One of the patients with nuclear cataract was homozygous for this allele. The in silico analysis revealed that D2D mutation results in a compact CRYAA mRNA secondary structure, while the wild type CRYAA mRNA has a weak or loose secondary structure. D2D mutation in the CRYAA gene may be an additional risk factor for progression of ARC.
Citations
Downloads
References
Kumar M, Agarwal T, Khokhar S, Kumar M, Kaur P, Roy TS, et al. Mutation screening and genotype phenotype correlation of alpha-crystallin, gamma-crystallin and GJA8 gene in congenital cataract. Mol Vis 2011;17:693-707.
Resnikoff S, Pascolini D, Mariotti SP, Pokharel GP. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ 2008;86(1):63-70. https://doi.org/10.2471/BLT.07.041210.
Gupta SK, Selvan VK, Agrawal SS, Saxena R. Advances in pharmacological strategies for the prevention of cataract development. Indian J Ophthalmol 2009;57(3):175-83. https://doi.org/10.4103/0301-4738.49390.
Koteiche HA, Claxton DP, Mishra S, Stein RA, McDonald ET, Mchaourab HS. Species-specific structural and functional divergence of α-crystallins: Zebrafish αBa- and rodent αA(ins)-crystallin encode activated chaperones. Biochemistry 2015;54(38):5949-58. https://doi.org/10.1021/acs.biochem.5b00678.
Horwitz J, Bova MF, Ding LL, Haley DA, Stewart PL. Lens alpha-crystallin: Function and structure. Eye (Lond) 1999;13(Pt 3b):403-8. https://doi.org/10.1038/eye.1999.114.
Beebe DC, Shui Y-B. Progress in preventing age-related cataract. In: Yorio T, Clark AF, Wax MB, editors. Ocular therapeutics. New York, NY: Academic Press; 2008. p. 143-66.
Spector A. Oxidative stress-induced cataract: Mechanism of action. FASEB J 1995;9(12):1173-82.
Hansen L, Yao W, Eiberg H, Kjaer KW, Baggesen K, Hejtmancik JF, et al. Genetic heterogeneity in microcornea-cataract: Five novel mutations in CRYAA, CRYGD, and GJA8. Invest Ophthalmol Vis Sci 2007;48(9):3937-44. https://doi.org/10.1167/iovs.07-0013.
Bhagyalaxmi SG, Srinivas P, Barton KA, Kumar KR, Vidyavathi M, Petrash JM, et al. A novel mutation (F71L) in alphaA-crystallin with defective chaperone-like function associated with age-related cataract. Biochim Biophys Acta 2009;1792(10):974-81. https://doi.org/10.1016/j.bbadis.2009.06.011.
Vicart P, Caron A, Guicheney P, Li Z, Prévost MC, Faure A, et al. A missense mutation in the alpha B-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 1998;20(1):92-5. https://doi.org/10.1038/1765.
Kerr CL, Zaveri MA, Robinson ML, Williams T, West-Mays JA. AP-2α is required after lens vesicle formation to maintain lens integrity. Dev Dyn 2014;243(10):1298-309. https://doi.org/10.1002/dvdy.24141.
Cvekl A, Duncan MK. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res 2007;26(6):555-97. https://doi.org/10.1016/j.preteyeres.2007.07.002.
Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. The longitudinal study of cataract study group. Arch Ophthalmol 1993;111(6):831-6. https://doi.org/10.1001/archopht.1993.01090060119035.
Pras E, Frydman M, Levy-Nissenbaum E, Bakhan T, Raz J, Assia EI, et al. A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Jewish Persian family. Invest Ophthalmol Vis Sci 2000;41(11):3511-5.
Devi RR, Yao W, Vijayalakshmi P, Sergeev YV, Sundaresan P, Hejtmancik JF. Crystallin gene mutations in Indian families with inherited pediatric cataract. Mol Vis 2008;14:1157-70.
Santana A, Waiswol M, Arcieri ES, Cabral de Vasconcellos JP, Barbosa de Melo M. Mutation analysis of CRYAA, CRYGC, and CRYGD associated with autosomal dominant congenital cataract in Brazilian families. Mol Vis 2009;15:793-800.
Chamary JV, Hurst LD. The price of silent mutations. Sci Am 2009;300(6):46-53. https://doi.org/10.1038/scientificamerican0609-46.
Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol 1987;196(4):947-50. https://doi.org/10.1016/0022-2836(87)90418-9.
Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol 1987;7(10):3438-45. https://doi.org/10.1128/MCB.7.10.3438.
Kozak M. A consideration of alternative models for the initiation of translation in eukaryotes. Crit Rev Biochem Mol Biol 1992;27(4-5):385-402. https://doi.org/10.3109/10409239209082567.
Kozak M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol Cell Biol 1989;9(11):5073-80. https://doi.org/10.1128/MCB.9.11.5073.
Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene 1999;234(2):187-208. https://doi.org/10.1016/S0378-1119(99)00210-3.
Muralidhar S, Becerra SP, Rose JA. Site-directed mutagenesis of adeno-associated virus type 2 structural protein initiation codons: Effects on regulation of synthesis and biological activity. J Virol 1994;68(1):170-6.
Grymek K, Łukasiewicz S, Faron-Góreckaa A, Tworzydlo M, Polit A, Dziedzicka-Wasylewska M. Role of silent polymorphisms within the dopamine D1 receptor associated with schizophrenia on D1-D2 receptor hetero-dimerization. Pharmacol Rep 2009;61(6):1024-33. https://doi.org/10.1016/S1734-1140(09)70164-1.
Downloads
Additional Files
Published
How to Cite
Accepted 2016-11-30
Published 2017-05-20









