Apocrine carcinoma of the breast: A brief update on the molecular features and targetable biomarkers
DOI:
https://doi.org/10.17305/bjbms.2016.1811Keywords:
breast cancer, special types, apocrine carcinoma, androgen receptor, biomarkers, targeted therapyAbstract
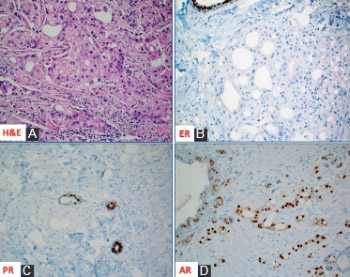
Apocrine carcinoma of the breast is a rare, primary breast cancer characterized by the apocrine morphology, estrogen receptor-negative and androgen receptor-positive profile with a frequent overexpression of Her-2/neu protein (~30%). Apart from the Her-2/neu target, advanced and/or metastatic apocrine carcinomas have limited treatment options. In this review, we briefly describe and discuss the molecular features and new theranostic biomarkers for this rare mammary malignancy. The importance of comprehensive profiling is highlighted due to synergistic and potentially antagonistic molecular events in the individual patients.
Citations
Downloads
References
Eusebi V, Millis R, Cattani M, Bussolati G, Azzopardi J. Apocrine carcinoma of the breast. A morphologic and immunocytochemical study. Am J Pathol 1986;123(3): 532-41.
Gatalica Z. Immunohistochemical analysis of apocrine breast lesions. Pathol Res Pract 1997;193(11-12):753-8.
Vranic S, Tawfik O, Palazzo J, Bilalovic N, Eyzaguirre E, Lee LM, et al. EGFR and HER-2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol 2010;23(5):644-53.
Vranic S, Schmitt F, Sapino A, Costa JL, Reddy S, Castro M, et al. Apocrine carcinoma of the breast: A comprehensive review. Histol Histopathol 2013;28(11):1393-1409.
Vranic S, Marchiò C, Castellano I, Botta C, Scalzo MS, Bender RP, et al. Immunohistochemical and molecular profiling of histologically defined apocrine carcinomas of the breast. Hum Pathol 2015;46(9):1350-9.
Mills AM, E Gottlieb C, M Wendroth S, M Brenin C, Atkins KA. Pure Apocrine Carcinomas Represent a Clinicopathologically Distinct Androgen Receptor-Positive Subset of Triple-Negative Breast Cancers. Am J Surg Pathol 2016;40(8):1109-16.
Bratthauer GL, Lininger RA, Man YG, Tavassoli FA. Androgen and estrogen receptor mRNA status in apocrine carcinomas. Diagn Mol Pathol 2002;11(2):113-8.
Vranic S, Gatalica Z, Deng H, Frkovic-Grazio S, Lee LM, Gurjeva O, et al. ER-α36 a novel isoform of ER-α66 is commonly over-expressed in apocrine and adenoid cystic carcinoma of the breast. J Clin Pathol 2011;64(1):54-7.
Costa LJ, Justino A, Gomes M, Alvarenga CA, Gerhard R, Vranic S, et al. Comprehensive genetic characterization of apocrine lesions of the breast. Cancer Res 2013;73(8):2013.
Naderi A, Meyer M, Dowhan DH. Cross-regulation between FOXA1 and ErbB2 signaling in estrogen receptor-negative breast cancer. Neoplasia 2012;14(4):283-96.
Naderi A, Hughes-Davies L. A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia 2008;10(6):542-8.
Naderi A, Meyer M. Prolactin-induced protein mediates cell invasion and regulates integrin signaling in estrogen receptor-negative breast cancer. Breast Cancer Res 2012;14(4):R111.
Lehmann-Che J, Hamy AS, Porcher R, Barritault M, Bouhidel F, Habuellelah H, et al. Molecular apocrine breast cancers are aggressive estrogen receptor negative tumors overexpressing either HER2 or GCDFP15. Breast Cancer Res 2013;15(3):R37.
Weisman PS, Ng CK, Brogi E, Eisenberg RE, Won HH, Piscuoglio S, et al. Genetic alterations of triple negative breast cancer by targeted next-generation sequencing and correlation with tumor morphology. Mod Pathol 2016;29(5):476-88.
Arce-Salinas C, Riesco-Martinez MC, Hanna W, Bedard P, Warner E. Complete Response of Metastatic Androgen Receptor-Positive Breast Cancer to Bicalutamide: Case Report and Review of the Literature. J Clin Oncol 2016;34(4):e21-4.
Bonnefoi H, Grellety T, Tredan O, Saghatchian M, Dalenc F, Mailliez A, et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann Oncol 2016;27(5):812-8.
Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res 2013;19(19):5505-12.
Lehmann BD, Bauer JA, Schafer JM, Pendleton CS, Tang L, Johnson KC, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res 2014;16(4):406. doi: 10.1186/s13058-014-0406-x.
Shi W, Jiang T, Nuciforo P, Hatzis C, Holmes E, Harbeck N, et al. Pathway level alterations rather than mutations in single genes predict response to HER2-targeted therapies in the neo-ALTTO trial. Ann Oncol 2016 Sep 29. pii: mdw434.
Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, et al. Programmed cell death (PD-1) and its ligand (PD-L1) expression in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 2014;23(12):2965-70.
Joneja U, Vranic S, Swensen J, Feldman R, Chen W, Kimbrough J, et al. Comprehensive profiling of metaplastic breast carcinomas reveals frequent overexpression of PD-L1. J Clin Pathol. 2016 Aug 16. pii: jclinpath-2016-203874. doi: 10.1136/jclinpath-2016-203874. [Epub ahead of print]
Downloads
Additional Files
Published
How to Cite
Accepted 2016-11-29
Published 2017-02-21









