Localization of trefoil factor family peptide 3 (TFF3) in epithelial tissues originating from the three germ layers of developing mouse embryo
DOI:
https://doi.org/10.17305/bjbms.2017.1838Keywords:
TFF3, digestive system, embryonic development, epidermis, epithelium, germ layers, immunohistochemistry, mice, respiratory system, trefoil factor, urogenital systemAbstract
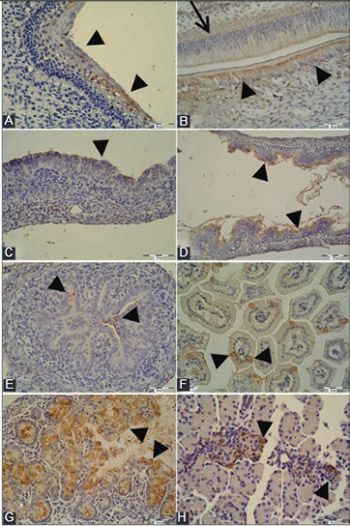
Trefoil factor family (TFF) peptides are involved in the maintenance of epithelial integrity and epithelial restitution. Mature epithelial tissues originate from different embryonic germ layers. The objective of this research was to explore the presence and localization of TFF3 peptide in mouse embryonic epithelia and to examine if the occurrence of TFF3 peptide is germ layer-dependent. Mouse embryos (14-18 days old) were fixed in 4% paraformaldehyde and embedded in paraffin. Immunohistochemistry was performed with affinity purified rabbit anti-TFF3 antibody, goat anti-rabbit biotinylated secondary antibody and streptavidin-horseradish peroxidase, followed by 3,3'-diaminobenzidine. TFF3 peptide was present in the gastric and intestinal mucosa, respiratory mucosa in the upper and lower airways, pancreas, kidney tubules, epidermis, and oral cavity. The presence and localization of TFF3 peptide was associated with the embryonic stage and tissue differentiation. TFF3 peptide distribution specific to the germ layers was not observed. The role of TFF3 peptide in cell migration and differentiation, immune response, and apoptosis might be associated with specific embryonic epithelial cells. TFF3 peptide may also be considered as a marker for mucosal maturation.
Citations
Downloads
References
Regalo G, Wright NA, Machado JC. Trefoil factors: From ulceration to neoplasia. Cell Mol Life Sci 2005;62(24):2910-5. https://doi.org/10.1007/s00018-005-5478-4.
Wright NA. Interaction of trefoil family factors with mucins: Clues to their mechanism of action? Gut 2001;48(3):293-4. https://doi.org/10.1136/gut.48.3.293.
Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci 2005;62(24):2932-8. https://doi.org/10.1007/s00018-005-5481-9.
Taupin D, Podolsky DK. Trefoil factors: Initiators of mucosal healing. Nat Rev Mol Cell Biol 2003;4(9):721-32. https://doi.org/10.1038/nrm1203.
Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A 2000;97(2):799-804. https://doi.org/10.1073/pnas.97.2.799.
Cook GA, Familari M, Thim L, Giraud AS. The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid tissues and participate in the immune response. FEBS Lett 1999;456(1):155-9. https://doi.org/10.1016/S0014-5793(99)00940-0.
Fu T, Znalesniak EB, Kalinski T, Möhle L, Biswas A, Salm F, et al. TFF peptides play a role in the immune response following oral infection of mice with Toxoplasma gondii. Eur J Microbiol Immunol (Bp) 2015;5(3):221-31. https://doi.org/10.1556/1886.2015.00028.
Hoffmann W. TFF (trefoil factor family) peptides and their potential roles for differentiation processes during airway remodeling. Curr Med Chem 2007;14(25):2716-9. https://doi.org/10.2174/092986707782023226.
Debata PR, Panda H, Supakar PC. Altered expression of trefoil factor 3 and cathepsin L gene in rat kidney during aging. Biogerontology 2007;8(1):25-30. https://doi.org/10.1007/s10522-006-9032-z.
Lubka M, Shah AA, Blin N, Baus-Loncar M. The intestinal trefoil factor (Tff3), also expressed in the inner ear, interacts with peptides contributing to apoptosis. J Appl Genet 2009;50(2):167-71. https://doi.org/10.1007/BF03195669.
Madsen J, Nielsen O, Tornøe I, Thim L, Holmskov U. Tissue localization of human trefoil factors 1, 2, and 3. J Histochem Cytochem 2007;55(5):505-13. https://doi.org/10.1369/jhc.6A7100.2007.
Schulze U, Sel S, Paulsen FP. Trefoil factor family peptide 3 at the ocular surface. A promising therapeutic candidate for patients with dry eye syndrome? Dev Ophthalmol 2010;45:1-11. https://doi.org/10.1159/000315014.
Barrera GJ, Sanchez G, Gonzalez JE. Trefoil factor 3 isolated from human breast milk downregulates cytokines (IL8 and IL6) and promotes human beta defensin (hBD2 and hBD4) expression in intestinal epithelial cells HT-29. Bosn J Basic Med Sci 2012;12(4):256-64.
Hoffmann W, Jagla W, Wiede A. Molecular medicine of TFF-peptides: From gut to brain. Histol Histopathol 2001;16(1):319-34.
Rösler S, Haase T, Claassen H, Schulze U, Schicht M, Riemann D, et al. Trefoil factor 3 is induced during degenerative and inflammatory joint disease, activates matrix metalloproteinases, and enhances apoptosis of articular cartilage chondrocytes. Arthritis Rheum 2010;62(3):815-25. https://doi.org/10.1002/art.27295.
Huang YG, Li YF, Wang LP, Zhang Y. Aberrant expression of trefoil factor 3 is associated with colorectal carcinoma metastasis. J Cancer Res Ther 2013;9(3):376-80. https://doi.org/10.4103/0973-1482.119308.
Pandey V, Wu ZS, Zhang M, Li R, Zhang J, Zhu T, et al. Trefoil factor 3 promotes metastatic seeding and predicts poor survival outcome of patients with mammary carcinoma. Breast Cancer Res 2014;16(5):429. https://doi.org/10.1186/s13058-014-0429-3.
Xiao L, Liu YP, Xiao CX, Ren JL, Guleng B. Serum TFF3 may be a pharamcodynamic marker of responses to chemotherapy in gastrointestinal cancers. BMC Clin Pathol 2014;14:26. https://doi.org/10.1186/1472-6890-14-26.
Kjellev S, Thim L, Pyke C, Poulsen SS. Cellular localization, binding sites, and pharmacologic effects of TFF3 in experimental colitis in mice. Dig Dis Sci 2007;52(4):1050-9. DOI: 10.1007/s10620-006-9256-4.
Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 1996;274(5285):262-5. https://doi.org/10.1126/science.274.5285.262.
Aamann L, Vestergaard EM, Grønbæk H. Trefoil factors in inflammatory bowel disease. World J Gastroenterol 2014;20(12):3223-30. https://doi.org/10.3748/wjg.v20.i12.3223.
Viby NE, Pedersen L, Lund TK, Kissow H, Backer V, Nexø E, et al. Trefoil factor peptides in serum and sputum from subjects with asthma and COPD. Clin Respir J 2015;9(3):322-9. https://doi.org/10.1111/crj.12146.
Lin J, Sun Z, Zhang W, Liu H, Shao D, Ren Y, et al. Protective effects of intestinal trefoil factor (ITF) on gastric mucosal epithelium through activation of extracellular signal-regulated kinase 1/2 (ERK1/2). Mol Cell Biochem 2015;404(1-2):263-70. https://doi.org/10.1007/s11010-015-2386-2.
Baus-Loncar M, Giraud AS. Multiple regulatory pathways for trefoil factor (TFF) genes. Cell Mol Life Sci 2005;62(24):2921-31. https://doi.org/10.1007/s00018-005-5480-x.
Kinoshita K, Taupin DR, Itoh H, Podolsky DK. Distinct pathways of cell migration and antiapoptotic response to epithelial injury: Structure-function analysis of human intestinal trefoil factor. Mol Cell Biol 2000;20(13):4680-90.
https://doi.org/10.1128/MCB.20.13.4680-4690.2000.
Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med 2002;8(10):1089-97. https://doi.org/10.1038/nm763.
Rivat C, Rodrigues S, Bruyneel E, Piétu G, Robert A, Redeuilh G, et al. Implication of STAT3 signaling in human colonic cancer cells during intestinal trefoil factor 3 (TFF3) and vascular endothelial growth factor-mediated cellular invasion and tumor growth. Cancer Res 2005;65(1):195-202.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell 1999;98(3):295-303.
https://doi.org/10.1016/S0092-8674(00)81959-5.
Efstathiou JA, Noda M, Rowan A, Dixon C, Chinery R, Jawhari A, et al. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci U S A 1998;95(6):3122-7. https://doi.org/10.1073/pnas.95.6.3122.
Gilbert SF. Developmental Biology. 6th ed. Sunderland: Sinauer Associates; 2000.
Belovari T, Bijelic N, Tolušic Levak M, Baus Loncar M. Trefoil factor family peptides TFF1 and TFF3 in the nervous tissues of developing mouse embryo. Bosn J Basic Med Sci 2015;15(1):33-7. https://doi.org/10.17305/bjbms.2015.251.
Bijelic N, Belovari T, Baus Loncar M. Trefoil factor family protein 3 (TFF3) is present in cartilage during endochondral ossification in the developing mouse fetus. Acta Histochem 2013;115(3):204-8. https://doi.org/10.1016/j.acthis.2012.06.007.
Familari M, Cook GA, Taupin DR, Marryatt G, Yeomans ND, Giraud AS. Trefoil peptides are early markers of gastrointestinal maturation in the rat. Int J Dev Biol 1998;42(6):783-9.
Hinz M, Schwegler H, Chwieralski CE, Laube G, Linke R, Pohle W, et al. Trefoil factor family (TFF) expression in the mouse brain and pituitary: Changes in the developing cerebellum. Peptides 2004;25(5):827-32. https://doi.org/10.1016/j.peptides.2004.01.020.
Otto WR, Patel K. Trefoil factor family (TFF)-domain peptides in the mouse: Embryonic gastrointestinal expression and wounding response. Anat Embryol (Berl) 1999;199(6):499-508. https://doi.org/10.1007/s004290050247.
Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002;129(11):2619-28.
Bossenmeyer-Pourié C, Kannan R, Ribieras S, Wendling C, Stoll I, Thim L, et al. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J Cell Biol 2002;157(5):761-70. https://doi.org/10.1083/jcb200108056.
Karam SM, Tomasetto C, Rio MC. Trefoil factor 1 is required for the commitment programme of mouse oxyntic epithelial progenitors. Gut 2004;53(10):1408-15. https://doi.org/10.1136/gut.2003.031963.
Guppy NJ, El-Bahrawy ME, Kocher HM, Fritsch K, Qureshi YA, Poulsom R, et al. Trefoil factor family peptides in normal and diseased human pancreas. Pancreas 2012;41(6):888-96. https://doi.org/10.1097/MPA.0b013e31823c9ec5.
Sotiropoulou PA, Blanpain C. Development and homeostasis of the skin epidermis. Cold Spring Harb Perspect Biol 2012;4(7):a008383. https://doi.org/10.1101/cshperspect.a008383.
Hanby AM, McKee P, Jeffery M, Grayson W, Dublin E, Poulsom R, et al. Primary mucinous carcinomas of the skin express TFF1, TFF3, estrogen receptor, and progesterone receptors. Am J Surg Pathol 1998;22(9):1125-31.
https://doi.org/10.1097/00000478-199809000-00012.
Paunel-Görgülü AN, Franke AG, Paulsen FP, Dünker N. Trefoil factor family peptide 2 acts pro-proliferative and pro-apoptotic in the murine retina. Histochem Cell Biol 2011;135(5):461-73. https://doi.org/10.1007/s00418-011-0810-6.
Lubka M, Müller M, Baus-Loncar M, Hinz M, Blaschke K, Hoffmann W, et al. Lack of Tff3 peptide results in hearing impairment and accelerated presbyacusis. Cell Physiol Biochem 2008;21(5-6):437-44. https://doi.org/10.1159/000129636.
Monk M, Holding C. Human embryonic genes re-expressed in cancer cells. Oncogene 2001;20(56):8085-91. https://doi.org/10.1038/sj.onc.1205088.
Downloads
Additional Files
Published
How to Cite
Accepted 2017-02-02
Published 2017-08-20









