Relationship between lymphovascular invasion and clinicopathological features of papillary thyroid carcinoma
DOI:
https://doi.org/10.17305/bjbms.2017.1924Keywords:
Papillary thyroid carcinoma, clinicopathological features, lymphovascular, PTC, LVI, lymphovascular invasion, BRAFV600E mutationAbstract
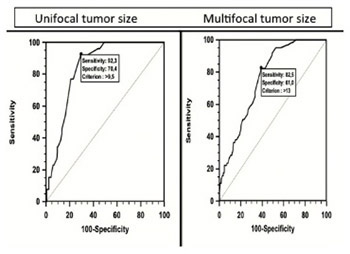
Lymphovascular invasion (LVI) is an important prognostic factor in various solid tumors, however, data on the association between LVI and thyroid carcinomas are limited. In this study, we evaluated the relationship between LVI and clinicopathological features of papillary thyroid carcinoma (PTC). Six hundred seventy-eight patients diagnosed with PTC between 2012 and 2015 were included into the study. Patients were classified based on the presence or absence of LVI. Gender, age, ultrasonography (US), tumor size and multifocality, BRAFV600E mutation, perineural and capsular invasion, extrathyroid extension (ETE), nodal metastasis, and recurrences were evaluated, and risk analysis was performed for each parameter. The number of patients with LVI [LVI (+)] was 63, while the number of patients without LVI [LVI (-)] was 615. The female/male ratio was 564/114. LVI was present in 18.4% of male patients and in 7.4 % of female patients. In the age group between 17-25 years LVI was detected in 6/13 patients, and this result was statistically significant compared to other age groups (p = 0.004). Suspicious lymph nodes upon US, perineural or capsular invasion, ETE, tumor size, and nodal metastasis were significantly more frequent in LVI (+) group (p < 0.001). The frequency of BRAFV600E mutation was also significantly higher in LVI (+) group (p < 0.001). Overall, the presence of LVI was associated with gender, tumor size, age, lymph node metastasis, pathological lymph nodes, perineural and capsular invasion, ETE, and BRAFV600E mutation. These results suggest that in PTC patients undergoing thyroidectomy, the presence of LVI should be considered as an indicator of aggressive clinicopathological features and those patients should be followed up carefully for recurrences and metastasis.
Citations
Downloads
References
Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011;21(2):125-34. https://doi.org/10.1089/thy.2010.0021.
Lang BH, Yih PCL, Shek TWH, Wan KY, Wong KP, Lo CY. Factors affecting the adequacy of lymph node yield in prophylactic unilateral central neck dissection for papillary thyroid carcinoma. J Surg Oncol 2012;106(8):966-71. https://doi.org/10.1002/jso.23201.
American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016 [cited 2016 November 10]. Available from: https://old.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf.
Cooper D, Doherty G, Haugen B, Kloos R, Lee S, Mandel S, et al. American Thyroid Association (ATA) guidelines task force on thyroid nodules and differentiated thyroid cancer. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19(11):1167-214. https://doi.org/10.1089/thy.2009.0110.
Dralle H, Machens A. Surgical approaches in thyroid cancer and lymph-node metastases. Best Pract Res Clin Endocrinol Metab 2008;22(6):971-87. https://doi.org/10.1016/j.beem.2008.09.018.
Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: A review and comparison. Ann Surg 2007;245(3):366-78. https://doi.org/10.1097/01.sla.0000250445.92336.2a.
Hay ID. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am 1990;19(3):545-76.
Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 1988;104(6):947-53.
Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1993;114(6):1050-7.
Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer 1979;15(8):1033-41.
https://doi.org/10.1016/0014-2964(79)90291-3.
Cady B. Hayes Martin Lecture. Our AMES is true: how an old concept still hits the mark: or, risk group assignment points the arrow to rational therapy selection in differentiated thyroid cancer. Am J Surg 1997;174(5):462-8.
https://doi.org/10.1016/S0002-9610(97)00162-1.
Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Prognostic factors in papillary and follicular thyroid carcinoma: Their implications for cancer staging. Ann Surg Oncol 2007;14(2):730-8. https://doi.org/10.1245/s10434-006-9207-5.
Duntas L, Grab-Duntas BM. Risk and prognostic factor for differentiated thyroid cancer. Hell J Nucl Med 2006;9(3):156-62.
Santos T, Estêvão R, Antunes L, Certal V, Silva JC, Monteiro E. Clinical and histopathological prognostic factors in locoregional advanced laryngeal cancer. J Laryngol Otol 2016;130(10):948-53. https://doi.org/10.1017/S002221511600880X.
Nathenson MJ, Ravi V, Fleming N, Wang WL, Conley A. Uterine Adenosarcoma: A review. Curr Oncol Rep 2016;18(11):68. https://doi.org/10.1007/s11912-016-0552-7.
Kommoss F, Kommoss F, Grevenkamp F, Bunz A-K, Taran F-A, Fend F, et al. L1CAM: Amending the "low-risk" category in endometrial carcinoma. J Cancer Res Clin Oncol 2016;143(2):255-262.
Al-Sukhni E, Attwood K, Gabriel EM, LeVea CM, Kanehira K, Nurkin SJ. Lymphovascular and perineural invasion are associated with poor prognostic features and outcomes in colorectal cancer: A retrospective cohort study. Int J Surg 2017;37:42-9. https://doi.org/10.1016/j.ijsu.2016.08.528.
Wei YS, Yao DS, Long Y. Evaluation of the association between perineural invasion and clinical and histopathological features of cervical cancer. Mol Clin Oncol 2016;5(3):307-11. https://doi.org/10.3892/mco.2016.941.
Min KW, Kim DH, Son BK, Kim EK, Ahn SB, Kim SH, et al. Invasion depth measured in millimeters is a predictor of survival in patients with distal bile duct cancer: Decision tree approach. World J Surg 2017;41(1):232-40. https://doi.org/10.1007/s00268-016-3687-7.
Barbera L, Gien LT, Sutradhar R, Thomas G, Covens A, Elit L, et al. The added value of pathology review in vulva cancer: Results from a population-based cohort study. Int J Gynecol Pathol 2017;36(2):107-10. DOI: 10.1097/PGP.0000000000000313.
Ieni A, Barresi V, Cardia R, Licata L, Di Bari F, Benvenga S, et al. The micropapillary/hobnail variant of papillary thyroid carcinoma: A review of series described in the literature compared to a series from one southern Italy pathology institution. Rev Endocr Metab Disord 2016 Nov 28. [Epub ahead of print].
https://doi.org/10.1007/s11154-016-9398-4.
Xu B, Wang L, Tuttle RM, Ganly I, Ghossein R. Prognostic impact of extent of vascular invasion in low-grade encapsulated follicular cell-derived thyroid carcinomas: A clinicopathologic study of 276 cases. Hum Pathol 2015;46(12):1789-98. https://doi.org/10.1016/j.humpath.2015.08.015.
Liu L, Chang JW, Jung SN, Park HS, Oh T, Lim YC, et al. Clinical implications of the extent of BRAFV600E alleles in patients with papillary thyroid carcinoma. Oral Oncol 2016;62:72-7. https://doi.org/10.1016/j.oraloncology.2016.10.005.
Walts AE, Mirocha JM, Bose S. Follicular variant of papillary thyroid carcinoma (FVPTC): Histological features, BRAF V600E mutation, and lymph node status. J Cancer Res Clin Oncol 2015;141(10):1749-56.
https://doi.org/10.1007/s00432-015-1939-9.
Pontius LN, Youngwirth LM, Thomas SM, Scheri RP, Roman SA, Sosa JA. Lymphovascular invasion is associated with survival for papillary thyroid cancer. Endocr Relat Cancer 2016;23(7):555-62. https://doi.org/10.1530/ERC-16-0123.
Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol 2011;24(12):1545-52. https://doi.org/10.1038/modpathol.2011.119.
Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid 2016;26(11):1541-52. https://doi.org/10.1089/thy.2016.0100.
Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Bethesda, MD: National Cancer Institute; 2009.
Can N, Tastekin E, Ozyilmaz F, Sezer YA, Guldiken S, Sut N, et al. Histopathological evidence of lymph node metastasis in papillary thyroid carcinoma. Endocr Pathol 2015;26(3):218-28. https://doi.org/10.1007/s12022-015-9382-7.
Yildirim E. A model for predicting outcomes in patients with differentiated thyroid cancer and model performance in comparison with other classification systems. J Am Coll Surg 2005;200(3):378-92. https://doi.org/10.1016/j.jamcollsurg.2004.10.031.
Akslen LA. Prognostic importance of histologic grading in papillary thyroid carcinoma. Cancer 1993;72(9):2680-5. https://doi.org/10.1002/1097-0142(19931101)72:9<2680::AID-CNCR2820720926>3.0.CO;2-D.
Sebastian SO, Gonzalez JR, Paricio PP, Perez JS, Flores DP, Madrona AP, et al. Papillary thyroid carcinoma: Prognostic index for survival including the histological variety. Arch Surg 2000;135(3):272-7. https://doi.org/10.1001/archsurg.135.3.272.
Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: Addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery 2004;135(2):139-48. https://doi.org/10.1016/S0039-6060(03)00384-2.
Song YJ, Shin SH, Cho JS, Park MH, Yoon JH, Jegal YJ. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J Breast Cancer 2011;14(3):198-203. https://doi.org/10.4048/jbc.2011.14.3.198.
Khwaja SS, Ivanovich J, DeWees TA, Ochoa L, Mullen DF, Thomas M, et al. Lymphovascular space invasion and lack of down staging after neoadjuvant chemotherapy are strong predictors of adverse outcome in young women with locally advanced breast cancer. Cancer Med 2016;5(2):230-8. https://doi.org/10.1002/cam4.586.
Gardner RE, Tuttle RM, Burman KD, Haddady S, Truman C, Sparling YH, et al. Prognostic importance of vascular invasion in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 2000;126(3):309-12. https://doi.org/10.1001/archotol.126.3.309.
Falvo L, Catania A, D'Andrea V, Marzullo A, Giustiniani MC, De Antoni E. Prognostic importance of histologic vascular invasion in papillary thyroid carcinoma. Ann Surg 2005;241(4):640-6. https://doi.org/10.1097/01.sla.0000157317.60536.08.
Kim JM, Kim TY, Kim WB, Gong G, Kim SC, Hong SJ, et al. Lymphovascular invasion is associated with lateral cervical lymph node metastasis in papillary thyroid carcinoma. Laryngoscope 2006;116(11):2081-5. https://doi.org/10.1097/01.mlg.0000242118.79647.a9.
Hsieh SH, Chen ST, Hsueh C, Chao TC, Lin JD. Gender-specific variation in the prognosis of papillary thyroid cancer TNM stages II to IV. Int J Endocrinol 2012;2012:379097. DOI: 10.1155/2012/379097.
Haymart MR. Understanding the relationship between age and thyroid cancer. Oncologist 2009;14(3):216-21. https://doi.org/10.1634/theoncologist.2008-0194.
Qu H, Sun Gr, Liu Y, He Qs. Clinical risk factors for central lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta‐analysis. Clin Endocrinol 2015;83(1):124-32. https://doi.org/10.1111/cen.12583.
Kim SY, Kwak JY, Kim EK, Yoon JH, Moon HJ. Association of preoperative US features and recurrence in patients with classic papillary thyroid carcinoma. Radiology 2015;277(2):574-83. https://doi.org/10.1148/radiol.2015142470.
Nam SY, Shin JH, Han BK, Ko EY, Ko ES, Hahn SY, et al. Preoperative ultrasonographic features of papillary thyroid carcinoma predictbiological behavior. J Clin Endocrinol Metab 2013;98(4):1476-82. https://doi.org/10.1210/jc.2012-4072.
Liu L, Chang JW, Jung SN, Park HS, Oh T, Lim YC, et al. Clinical implications of the extent of BRAFV600E alleles in patients with papillary thyroid carcinoma. Oral Oncol 2016;62:72-7. https://doi.org/10.1016/j.oraloncology.2016.10.005.
Kakarmath S, Heller HT, Alexander CA, Cibas ES, Krane JF, Barletta JA, et al. Clinical, sonographic, and pathological characteristics of RAS-positive versus BRAF-positive thyroid carcinoma. J Clin Endocrinol Metab 2016;101(12):4938-44.
https://doi.org/10.1210/jc.2016-2620.
Gambardella C, Tartaglia E, Nunziata A, Izzo G, Siciliano G, Cavallo F, et al. Clinical significance of prophylactic central compartment neck dissection in the treatment of clinically node-negative papillary thyroid cancer patients. World J Surg Oncol 2016;14(1):247. https://doi.org/10.1186/s12957-016-1003-5.
Conzo G, Mauriello C, Docimo G, Gambardella C, Thomas G, Cavallo F, et al. Clinicopathological pattern of lymph node recurrence of papillary thyroid cancer. Implications for surgery. Int J Surg 2014;12 Suppl 1:S194-7. https://doi.org/10.1016/j.ijsu.2014.05.010.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-02-12
Published 2017-05-20









