Enhancer of zeste homologue 2 (EZH2) expression in synovial sarcomas as a promising indicator of prognosis
DOI:
https://doi.org/10.17305/bjbms.2017.1938Keywords:
Enhancer of zeste homologue 2 (EZH2), immunohistochemistry, prognostic factors, synovial sarcoma, survival analysisAbstract
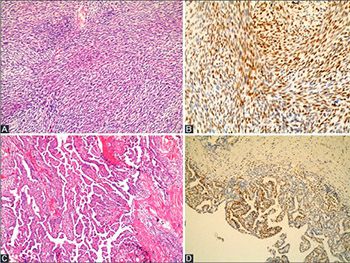
Synovial sarcoma (SS) is a type of soft-tissue sarcoma, often linked to poor survival. Although overexpression of enhancer of zeste homologue 2 (EZH2) has been associated with poor prognosis in different tumors, a few studies investigated this link in SS. Here, we analyzed the relationship between EZH2 expression and prognostic factors in SS. We included 29 patients with SS. Immunostaining of EZH2 was performed with (D2C9) XPTM Rabbit mAb antibody, and the results were classified as low EZH2 expression (negative or weak expression) and high EZH2 expression category (moderate or strong expression). Analysis of survival in relation to prognostic factors was performed with Kaplan-Meier survival curves and Cox proportional hazard regression analysis. Our sample included 19/29 female and 10/29 male patients, with age range 16-63 years. The tumor diameter ranged from 2 to 15 cm. Necrosis was observed in 15/29 cases. Sixteen cases had >10 mitoses per 50 high-power fields (HPFs). Out of 29 cases, 14 showed low and 15 had high EZH2 expression. Statistically significant results were obtained for the association between the presence of metastasis and necrosis (p = 0.042), high EZH2 expression and distant metastasis (p = 0.018), high EZH2 expression and necrosis (p = 0.016), and high EZH2 expression and the tumor size >5 cm versus tumor size ≤5 cm (p = 0.014). Patients with all of the following: the tumor size ≤5 cm, low EZH2 expression, and without necrosis and distant metastasis had significantly longer survival time. Our results are consistent with previous studies, suggesting that EZH2 overexpression is an indicator of poor prognosis in SS.
Citations
Downloads
References
Suurmeijer AJ, de Bruijn D, Geurts van Kessel A, Miettinen MM. Synovial sarcoma. In: Fletcher CD, Bridge JA, Hogendoor P, Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon: IARC; 2013. p. 213-5.
Goldblum JR, Folpe AL, Weiss SW, editors. Malignant soft tissue tumors of uncertain type. Enzinger and Weiss's Soft Tissue Tumors. 6th ed. Philadelphia, PA: Saunders; 2013. p. 1052-70.
Krieg AH, Hefti F, Speth BM, Jundt G, Guillou L, Exner UG, et al. Synovial sarcomas usually metastasize after >5 years: A multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol 2011;22(2):458-67. https://doi.org/10.1093/annonc/mdq394.
Lamm W, Schur S, Köstler WJ, Amann G, Pokrajac B, Funovics P, et al. Initially localized synovial sarcoma in adults: A retrospective single-center analysis of 26 patients registered at the Department of Oncology, University of Vienna between 2004 and 2013. Oncology 2014;87(1):48-57. https://doi.org/10.1159/000363185.
Machen SK, Easley KA, Goldblum JR. Synovial sarcoma of the extremities: A clinicopathologic study of 34 cases, including semi-quantitative analysis of spindled, epithelial, and poorly differentiated areas. Am J Surg Pathol 1999;23(3):268-75. https://doi.org/10.1097/00000478-199903000-00004.
Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol 2007;31(2):240-6. https://doi.org/10.1097/01.pas.0000213330.71745.39.
Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: A whole section study of 163 soft tissue and bone neoplasms. Mod Pathol 2009;22(7):872-8. https://doi.org/10.1038/modpathol.2009.47.
van de Rijn M, Barr FG, Xiong QB, Hedges M, Shipley J, Fisher C. Poorly differentiated synovial sarcoma: An analysis of clinical, pathologic, and molecular genetic features. Am J Surg Pathol 1999;23(1):106-12. https://doi.org/10.1097/00000478-199901000-00012.
Bergh P, Meis-Kindblom JM, Gherlinzoni F, Berlin O, Bacchini P, Bertoni F, et al. Synovial sarcoma: Identification of low and high risk groups. Cancer 1999;85(12):2596-607. https://doi.org/10.1002/(SICI)1097-0142(19990615)85:12<2596::AID-CNCR16>3.0.CO;2-K.
Trassard M, Le Doussal V, Hacène K, Terrier P, Ranchère D, Guillou L, et al. Prognostic factors in localized primary synovial sarcoma: A multicenter study of 128 adult patients. J Clin Oncol 2001;19(2):525-34. https://doi.org/10.1200/JCO.2001.19.2.525.
Lewis JJ, Antonescu CR, Leung DH, Blumberg D, Healey JH, Woodruff JM, et al. Synovial sarcoma: A multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol 2000;18(10):2087-94. https://doi.org/10.1200/JCO.2000.18.10.2087.
Sultan I, Rodriguez-Galindo C, Saab R, Yasir S, Casanova M, Ferrari A. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: An analysis of 1268 patients. Cancer 2009;115(15):3537-47. https://doi.org/10.1002/cncr.24424.
Yamaga K, Osaki M, Kidani K, Shomori K, Yoshida H, Ito H. High expression of enhancer of zeste homologue 2 indicates poor prognosis in patients with soft tissue sarcomas. Mol Med Rep 2008;1(5):633-9. https://doi.org/10.3892/mmr_00000004.
Chase A, Cross NC. Aberrations of EZH2 in cancer. Clin Cancer Res 2011;17(9):2613-8. https://doi.org/10.1158/1078-0432.CCR-10-2156.
Chang CJ, Hung MC. The role of EZH2 in tumour progression. Br J Cancer 2012;106(2):243-7. https://doi.org/10.1038/bjc.2011.551.
Sasaki H, Setoguchi T, Matsunoshita Y, Gao H, Hirotsu M, Komiya S. The knock-down of overexpressed EZH2 and BMI-1 does not prevent osteosarcoma growth. Oncol Rep 2010;23(3):677-84.
Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 2003;22(20):5323-35. https://doi.org/10.1093/emboj/cdg542.
Kanno R, Janakiraman H, Kanno M. Epigenetic regulator polycomb group protein complexes control cell fate and cancer. Cancer Sci 2008;99(6):1077-84. https://doi.org/10.1111/j.1349-7006.2008.00797.x.
Alford SH, Toy K, Merajver SD, Kleer CG. Increased risk for distant metastasis in patients with familial early-stage breast cancer and high EZH2 expression. Breast Cancer Res Treat 2012;132(2):429-37. https://doi.org/10.1007/s10549-011-1591-2.
Sudo T, Utsunomiya T, Mimori K, Nagahara H, Ogawa K, Inoue H, et al. Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J Cancer 2005;92(9):1754-8. https://doi.org/10.1038/sj.bjc.6602531.
Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 2003;423(6937):255-60. https://doi.org/10.1038/nature01572.
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002;419(6907):624-9. https://doi.org/10.1038/nature01075.
Changchien YC, Tátrai P, Papp G, Sápi J, Fónyad L, Szendroi M, et al. Poorly differentiated synovial sarcoma is associated with high expression of enhancer of zeste homologue 2 (EZH2). J Transl Med 2012;10:216. https://doi.org/10.1186/1479-5876-10-216.
Kawano S, Grassian AR, Tsuda M, Knutson SK, Warholic NM, Kuznetsov G, et al. Preclinical evidence of anti-tumor activity induced by EZH2 inhibition in human models of synovial sarcoma. PLoS One 2016;11(7):1-22. https://doi.org/10.1371/journal.pone.0158888.
Shen JK, Cote GM, Gao Y, Choy E, Mankin HJ, Hornicek FJ, et al. Targeting EZH2-mediated methylation of H3K27 inhibits proliferation and migration of synovial sarcoma in vitro. Sci Rep 2016;6:25239. https://doi.org/10.1038/srep25239.
Ferrari A, Gronchi A, Casanova M, Meazza C, Gandola L, Collini P, et al. Synovial sarcoma: A retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 2004;101(3):627-34. https://doi.org/10.1002/cncr.20386.
Guillou L, Benhattar J, Bonichon F, Gallagher G, Terrier P, Stauffer E, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: A multicenter, retrospective analysis. J Clin Oncol 2004;22(20):4040-50. https://doi.org/10.1200/JCO.2004.11.093.
ten Heuvel SE, Hoekstra HJ, Bastiaannet E, Suurmeijer AJ. The classic prognostic factors tumor stage, tumor size, and tumor grade are the strongest predictors of outcome in synovial sarcoma: No role for SSX fusion type or ezrin expression. Appl Immunohistochem Mol Morphol 2009;17(3):189-95. https://doi.org/10.1097/PAI.0b013e31818a6f5c.
Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer 1982;50(2):345-52. https://doi.org/10.1002/1097-0142(19820715)50:2<345::AID-CNCR2820500231>3.0.CO;2-Y.
Paulino AC. Synovial sarcoma prognostic factors and patterns of failure. Am J Clin Oncol 2004;27(2):122-7. https://doi.org/10.1097/01.coc.0000047130.91699.DC.
Campbell C, Gallagher J, Dickinson I. Synovial sarcoma - Towards a simplified approach to prognosis. ANZ J Surg 2004;74(9):727-31.
https://doi.org/10.1111/j.1445-1433.2004.03144.x.
Wright PH, Sim FH, Soule EH, Taylor WF. Synovial sarcoma. J Bone Joint Surg Am 1982;64(2):112-22. https://doi.org/10.2106/00004623-198264010-00016.
Canter RJ, Qin LX, Maki RG, Brennan MF, Ladanyi M, Singer S. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res 2008;14(24):8191-7. DOI: 10.1158/1078-0432.CCR-08-0843.
Ferrari A, Bisogno G, Alaggio R, Cecchetto G, Collini P, Rosolen A, et al. Synovial sarcoma of children and adolescents: The prognostic role of axial sites. Eur J Cancer 2008;44(9):1202-9. https://doi.org/10.1016/j.ejca.2008.03.016.
Soole F, Maupain C, Defachelles AS, Taque S, Minard-Colin V, Bergeron C, et al. Synovial sarcoma relapses in children and adolescents: Prognostic factors, treatment, and outcome. Pediatr Blood Cancer 2014;61(8):1387-93. https://doi.org/10.1002/pbc.25001.
Downloads
Additional Files
Published
How to Cite
Accepted 2017-02-18
Published 2017-11-20









