Prevalence of F5 1691G>A, F2 20210G>A, and MTHFR 677C>T polymorphisms in Bosnian women with pregnancy loss
DOI:
https://doi.org/10.17305/bjbms.2017.1954Keywords:
Pregnancy loss, risk factors, polymorphisms, thrombophilia, F5, F2, MTHFRAbstract
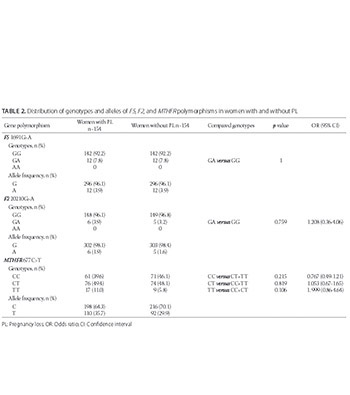
The relationship between genetic risk factors of thrombophilia and pregnancy loss (PL) is being discussed. The focus has been on F5 1691G>A, F2 20210G>A, and MTHFR 677C>T polymorphisms that may predispose women to microthrombosis during the stages of embryo implantation and placentation. Although, the frequencies of these polymorphisms were reported in different populations, such studies have not yet been performed in Bosnian population. In this study, we determined the prevalence of F5 G>A (rs6025), F2 G>A (rs1799963) and MTHFR C>T (rs1801133) polymorphisms in Bosnian women. A total of 154 women with PL, mean age 33 (±5.4) years, were enrolled in the study. As a control group, 154 mothers [mean age 31.4 (±6.7) years] with at least one live-born child were included. We used real-time polymerase chain reaction (PCR) to determine the frequencies of F5 G>A and F2 G>A genotypes, and PCR-restriction fragment length polymorphism (RFLP) for analyzing MTHFR C>T genotypes. The frequency of heterozygotes for F5 and F2 was significantly higher in women with venous thrombosis (VT) compared to women without VT (p = 0.047 and p = 0.001, respectively). There was no significant difference in the distribution of MTHFR genotypes and alleles between these two groups. In addition, we observed no significant differences in the genotype and allele frequencies between the group with PL and control group, for all investigated polymorphisms. The allele frequencies for 1691A (F5), 20210A (F2), and 677T (MTHFR) reported in this study are consistent with the data obtained for other European countries, however, we were not able to confirm the association between the three polymorphisms and PL in Bosnian women.
Citations
Downloads
References
Walker ID. Thrombophilia in pregnancy. J Clin Pathol 2000;53(8):573-80. https://doi.org/10.1136/jcp.53.8.573.
WHO: Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October. Acta Obstet Gynecol Scand 1977;56(3):247-53.
Rodeghiero F, Tosetto A. Activated protein C resistance and factor V Leiden mutation are independent risk factors for venous thromboembolism. Ann Intern Med 1999;130(8):643-50. https://doi.org/10.7326/0003-4819-130-8-199904200-00004.
Adler G, Agnieszka G, Valjevac A, Czerska E, Kiseljakovic E, Salkic NN. Prevalence of genetic prothrombotic risk factors: 1691G > A FV, 20210G > A PT and 677C > T MTHFR mutations in the Bosnian population. Ann Hum Biol 2015;42(6):576-80. https://doi.org/10.3109/03014460.2014.968618.
Inbal A, Carp H. Defects in coagulation factor leading to recurrent pregnancy loss. In: Carp H, editor. Recurrent Pregnancy Loss. Causes Controversies and Treatment. UK: Informa Healthcare; 2007. p. 127-39. https://doi.org/10.3109/9780203931677.019.
Finan RR, Tamim H, Ameen G, Sharida HE, Rashid M, Almawi WY. Prevalence of factor V G1691A (factor V-Leiden) and prothrombin G20210A gene mutations in a recurrent miscarriage population. Am J Hematol 2002;71(4):300-5. https://doi.org/10.1002/ajh.10223.
Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet 2001;109(4):369-84. https://doi.org/10.1007/s004390100593.
Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996;88(10):3698-703.
Jadaon MM. Epidemiology of prothrombin G20210A mutation in the Mediterranean region. Mediterr J Hematol Infect Dis 2011;3(1):e2011054. https://doi.org/10.4084/mjhid.2011.037.
American College of Obstetricians and Gynecologists Women's Health Care Physicians. ACOG Practice Bulletin No. 138: Inherited thrombophilias in pregnancy. Obstet Gynecol 2013;122(3):706-17. https://doi.org/10.1097/01.AOG.0000433981.36184.4e.
Schmitz C, Lindpaintner K, Verhoef P, Gaziano JM, Buring J. Genetic polymorphism of methylenetetrahydrofolate reductase and myocardial infarction. A case-control study. Circulation 1996;94(8):1812-4. https://doi.org/10.1161/01.CIR.94.8.1812.
Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost 2003;29(2):125-30. https://doi.org/10.1055/s-2003-38897.
Slezak R, Laczmanski L, Karpinski P, Reszczynska-Slezak D. The role of 1691G>A (Leiden) mutation in Factor V gene, 20210G>A in prothrombin gene and 677C>T in MTHFR gene in etiology of early pregnancy loss. [Article in Polish]. Ginekol Pol 2011;82(6):446-50.
Reznikoff-Etievan MF, Cayol V, Carbonne B, Robert A, Coulet F, Milliez J. Factor V Leiden and G20210A prothrombin mutations are risk factors for very early recurrent miscarriage. BJOG 2001;108(12):1251-4. https://doi.org/10.1016/S0306-5456(01)00298-4.
Altintas A, Pasa S, Akdeniz N, Cil T, Yurt M, Ayyildiz O, et al. Factor V Leiden and G20210A prothrombin mutations in patients with recurrent pregnancy loss: Data from the southeast of Turkey. Ann Hematol 2007;86(10):727-31.
https://doi.org/10.1007/s00277-007-0327-1.
Djurovic J, Stojkovic O, Todorovic J, Brajic A, Obradovic S, Stankovic S, et al. Genetics of suspected thrombophilia in Serbian females with infertility, including three cases, homozygous for FII 20210A or FV 1691A mutations. Hum Fertil (Camb) 2017;20(2):132-9. https://doi.org/10.1080/14647273.2016.1255785.
Hussein AS, Darwish H, Shelbayeh K. Association between factor V Leiden mutation and poor pregnancy outcomes among Palestinian women. Thromb Res 2010;126(2):e78-82. https://doi.org/10.1016/j.thromres.2010.04.017
Carp H, Salomon O, Seidman D, Dardik R, Rosenberg N, Inbal A. Prevalence of genetic markers for thrombophilia in recurrent pregnancy loss. Hum Reprod 2002;17(6):1633-7. https://doi.org/10.1093/humrep/17.6.1633.
Rodger MA, Betancourt MT, Clark P, Lindqvist PG, Dizon-Townson D, Said J, et al. The association of factor V leiden and prothrombin gene mutation and placenta-mediated pregnancy complications: A systematic review and meta-analysis of prospective cohort studies. PLoS Med 2010;7(6):e1000292. https://doi.org/10.1371/journal.pmed.1000292.
Sehirali S, Inal MM, Yildirim Y, Balim Z, Kosova B, Karamizrak T, et al. Prothrombin G20210A mutation in cases with recurrent miscarriage: A study of the mediterranean population. Arch Gynecol Obstet 2005;273(3):170-3. https://doi.org/10.1007/s00404-005-0061-7.
Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: A meta-analysis. Lancet 2003;361(9361):901-8. https://doi.org/10.1016/S0140-6736(03)12771-7.
Creus M, Deulofeu R, Peñarrubia J, Carmona F, Balasch J. Plasma homocysteine and vitamin B12 serum levels, red blood cell folate concentrations, C677T methylenetetrahydrofolate reductase gene mutation and risk of recurrent miscarriage: A case-control study in Spain. Clin Chem Lab Med 2013;51(3):693-9. https://doi.org/10.1515/cclm-2012-0452.
Sotiriadis A, Vartholomatos G, Pavlou M, Kolaitis N, Dova L, Stefos T, et al. Combined thrombophilic mutations in women with unexplained recurrent miscarriage. Am J Reprod Immunol 2007;57(2):133-41. https://doi.org/10.1111/j.1600-0897.2006.00454.x.
Foka ZJ, Lambropoulos AF, Saravelos H, Karas GB, Karavida A, Agorastos T, et al. Factor V leiden and prothrombin G20210A mutations, but not methylenetetrahydrofolate reductase C677T, are associated with recurrent miscarriages. Hum Reprod 2000;15(2):458-62. https://doi.org/10.1093/humrep/15.2.458.
Robertson L, Wu O, Langhorne P, Twaddle S, Clark P, Lowe GD, et al. Thrombophilia in pregnancy: A systematic review. Br J Haematol 2006;132(2):171-96. https://doi.org/10.1111/j.1365-2141.2005.05847.x.
Biron-Andreani C, Schved JF, Daures JP. Factor V Leiden mutation and pregnancy-related venous thromboembolism: What is the exact risk? Results from a meta-analysis. Thromb Haemost 2006;96(1):14-8. https://doi.org/10.1160/th06-02-0086.
Van Dongen CJ, Vink R, Hutten BA, Buller HR, Prins MH. The incidence of recurrent venous thromboembolism after treatment with vitamin K antagonists in relation to time since first event: A meta-analysis. Arch Intern Med 2003;163(11):1285-93. https://doi.org/10.1001/archinte.163.22.2793-a.
Coriu L, Ungureanu R, Talmaci R, Uscatescu V, Cirstoiu M, Coriu D, et al. Hereditary thrombophilia and thrombotic events in pregnancy: Single-center experience. J Med Life 2014;7(4):567-71.
Hotoleanu C, Popp R, Trifa A. Factor V Leiden, prothrombin G20210A and MTHFR C677T mutations in Romanian patients with deep venous thrombosis. HVM Bioflux 2014;6(1):15-9.
Downloads
Additional Files
Published
How to Cite
Accepted 2017-04-01
Published 2017-11-20









