Epigenetic alterations of the Wnt signaling pathway in cancer: a mini review
DOI:
https://doi.org/10.17305/bjbms.2014.4.205Keywords:
Cancer, epigenetics, Wnt signaling pathwayAbstract
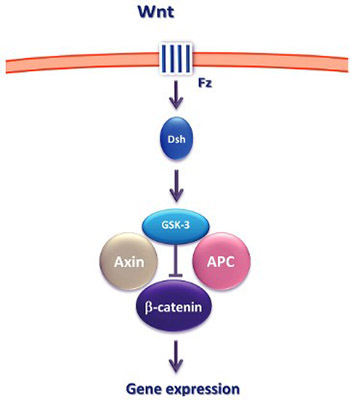
Epigenetic mechanisms play a crucial role in cellular proliferation, migration and differentiation in both normal and neoplastic development. One of the key signaling pathways whose components are altered through the epigenetic mechanisms is the Wnt signaling pathway. In this review, we briefly discuss the key concepts of epigenetics and focus on the recent advances in the Wnt signaling pathway research and its potential diagnostic and therapeutic implications.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2014-11-02
Published 2014-11-12









