A prominent lactate peak as a potential key magnetic resonance spectroscopy (MRS) feature of progressive multifocal leukoencephalopathy (PML): Spectrum pattern observed in three patients
DOI:
https://doi.org/10.17305/bjbms.2017.2092Keywords:
Progressive multifocal leukoencephalopathy, PML, magnetic resonance spectroscopy, MRS, lactic acid, JC virus, HIVAbstract
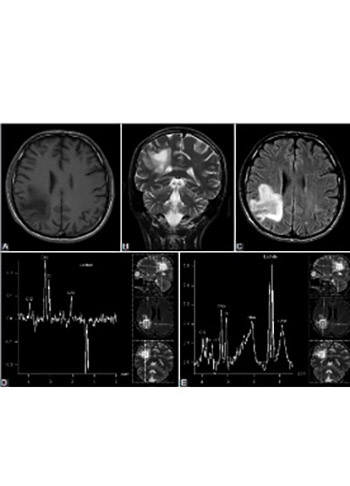
Progressive multifocal leukoencephalopathy (PML) is a rare, often fatal, opportunistic infection, associated with demyelinating process. PML is caused by John Cunningham (JC) polyomavirus, and predominantly affects patients with human immunodeficiency virus (HIV) infection or other immunocompromised patients. The purpose of this study was to determine the role of magnetic resonance spectroscopy (MRS) in establishing the diagnosis of PML. MRS with long and short echo time was performed in two patients with PML associated with HIV infection and in one PML patient associated with chronic lymphocytic leukemia. The most prominent peak on the obtained spectra was for lactate; it showed 2-3 times higher concentration of lactate compared to choline, almost 4-6 times higher lactate concentration compared to creatine, and 4-11 times higher lactate in comparison to N-acetylaspartate concentration. Similar spectrum pattern was observed in all patients. To the best of our knowledge, this is a new finding that might be useful in early diagnosis of PML. Nevertheless, further confirmation of our results is needed, since we analyzed the spectrum pattern only in three patients. Overall, our results could help in early detection of PML, especially in non-HIV patients, and thus prevent the fatal outcome of the disease. MRS could also be useful in detecting “tumefactive” demyelinating lesions in PML patients, associated with immune reconstitution inflammatory syndrome, to avoid misdiagnosis of neoplasm.
Citations
Downloads
References
Sharma SK, Soneja M, Ranjan S, Miglani S, Hari S, Sinha S, et al. Progressive multifocal leucoencephalopathy in HIV/AIDS: Observational study from a tertiary care centre in northern India. Indian J Med Res 2013;138(1):72-7.
Hurley RA, Ernst T, Khalili K, Del Valle L, Simone IL, Taber KH. Identification of HIV-associated progressive multifocal leukoencephalopathy: Magnetic resonance imaging and spectroscopy. J Neuropsychiatry Clin Neurosci 2003;15(1):1-6. https://doi.org/10.1176/jnp.15.1.1.
Kastrup O, Maschke M, Diener HC, Wanke I. Progressive multifocal leukoencephalopathy limited to the brain stem. Neuroradiology 2002;44(3):227-9.
https://doi.org/10.1007/s00234-001-0714-6.
Kozic D, Ostojic J, Bjelan M, Koprivšek K. The role of MR spectroscopy in neurooncology. Prilozi 2012;33(1):425-33.
Sweet TM, Del Valle L, Khalili K. Molecular biology and immunoregulation of human neurotropic JC virus in CNS. J Cell Physiol 2002;191(3):249-56. https://doi.org/10.1002/jcp.10096.
Holloway RG, Mushlin AI. Intracranial mass lesions in acquired immunodeficiency syndrome: Using decision analysis to determine the effectiveness of stereotactic brain biopsy. Neurology 1996;46(4):1010-5. https://doi.org/10.1212/WNL.46.4.1010.
Iranzo A, Moreno A, Pujol J, Martí-Fàbregas J, Domingo P, Molet J, et al. Proton magnetic resonance spectroscopy pattern of progressive multifocal leukoencephalopathy in AIDS. J Neurol Neurosurg Psychiatry 1999;66(4):520-3. https://doi.org/10.1136/jnnp.66.4.520.
Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: Consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80(15):1430-8. https://doi.org/10.1212/WNL.0b013e31828c2fa1.
Pavlovic AM, Bonaci-Nikolic B, Kozic D, Ostojic J, Abinun M, Svabic-Medjedovic T, et al. Progressive multifocal leukoencephalopathy associated with mycophenolate mofetil treatment in a woman with lupus and CD4 T-lymphocyte deficiency. Lupus 2012;21(1):100-2. https://doi.org/10.1177/0961203311416693.
Yoon JH, Bang OY, Kim HS. Progressive multifocal leukoencephalopathy in AIDS: Proton MR spectroscopy patterns of asynchronous lesions confirmed by serial diffusion-weighted imaging and apparent diffusion coefficient mapping. J Clin Neurol 2007;3(4):200-3. https://doi.org/10.3988/jcn.2007.3.4.200.
Aksamit AJ Jr. Progressive multifocal leukoencephalopathy. Continuum (Minneap Minn) 2012;18(6 Infectious Disease):1374-91. https://doi.org/10.1212/01.CON.0000423852.70641.de.
Dahlhaus S, Hoepner R, Chan A, Kleiter I, Adams O, Lukas C, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry 2013;84(10):1068-74. https://doi.org/10.1136/jnnp-2013-304897.
Davis MJ, Khan A, Royal W 3rd. Progressive multifocal leukoencephalopathy as the first manifestation of occult sarcoidosis: Case report and review of the literature. Neurologist 2013;19(1):26-9. https://doi.org/10.1097/NRL.0b013e31827c6c3d.
Christakis PG, Okin D, Huttner AJ, Baehring JM. Progressive multifocal leukoencephalopathy in an immunocompetent patient. J Neurol Sci 2013;326(1-2):107-10. https://doi.org/10.1016/j.jns.2013.01.010.
Sahraian MA, Radue EW, Eshaghi A, Besliu S, Minagar A. Progressive multifocal leukoencephalopathy: A review of the neuroimaging features and differential diagnosis. Eur J Neurol 2012;19(8):1060-9. https://doi.org/10.1111/j.1468-1331.2011.03597.x.
Shah R, Bag AK, Chapman PR, Curé JK. Imaging manifestations of progressive multifocal leukoencephalopathy. Clin Radiol 2010;65(6):431-9. https://doi.org/10.1016/j.crad.2010.03.001.
Gheuens S, Ngo L, Wang X, Alsop DC, Lenkinski RE, Koralnik IJ. Metabolic profile of PML lesions in patients with and without IRIS: An observational study. Neurology 2012;79(10):1041-8. https://doi.org/10.1212/WNL.0b013e318268465b.
Katz-Brull R, Lenkinski RE, Du Pasquier RA, Koralnik IJ. Elevation of myoinositol is associated with disease containment in progressive multifocal leukoencephalopathy. Neurology 2004;63(5):897-900. https://doi.org/10.1212/01.WNL.0000137420.58346.9F.
Kozic D, Nagulic M, Samardzic M, Ostojic J, Rasulic L, Cvetkovic-Dozic D. Intrapontine malignant nerve sheath tumor: MRI and MRS features. Acta Neurol Belg 2008;108(2):67-71.
Cuvinciuc V, Martin-Blondel G, Marchou B, Bonneville F. Proton MR spectroscopy of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome. AJNR Am J Neuroradiol 2010;31(8):E69-70. https://doi.org/10.3174/ajnr.A2160.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-05-10
Published 2017-11-20









