ABCB1 3435C>T and 2677G>T/A polymorphisms in Polish and Bosnian patients with Crohn’s disease – A preliminary report
DOI:
https://doi.org/10.17305/bjbms.2017.2172Keywords:
ABCB1, Crohn’s disease, allele frequency, Poland, Bosnia and Herzegovina, 3435C>T, 2677G>T/A, single nucleotide polymorphism, SNPAbstract
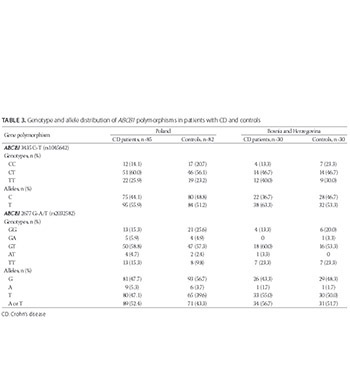
The role of ABCB1 single nucleotide polymorphisms (SNPs) in the development of Crohn’s disease (CD) remains unclear. Due to inconsistent results of several European population-based studies and limited information on populations from Poland and Bosnia and Herzegovina (BH), we conducted a preliminary association study of two main ABCB1 SNPs and CD. ABCB1 3435C>T and 2677G>T/A SNPs were analyzed in Polish and Bosnian patients with CD (n = 85 and n = 30, respectively) and controls (n = 82 and n = 30, respectively) using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) for 3435C>T and allele-specific PCR for 2677G>A/T SNP. A deviation from Hardy-Weinberg equilibrium was found for both SNPs in Polish patients with CD, and for 2677G>A/T in Polish control group. The allele and genotype frequencies of the two ABCB1 SNPs were not significantly different between the CD patients and controls in both populations (p > 0.05). Similarly, the genotype distribution of 3435C>T and 2677G>T/A SNPs was not significantly different between Polish and Bosnian patients with CD (p > 0.05). At least one mutated ABCB1 allele was carried by 97.7% of Polish and 90.0% of Bosnian patients with CD. No association was found between the ABCB1 SNPs and CD in the two populations. In conclusion, the two ABCB1 SNPs may not contribute to CD susceptibility in the populations of Poland and BH. Further studies with larger samples in both populations are warranted.
Citations
Downloads
References
Baumgart DC, Sandborn WJ. Crohn's disease. Lancet 2012;380(9853):1590-605. https://doi.org/10.1016/S0140-6736(12)60026-9.
de Lange KM, Barrett JC. Understanding inflammatory bowel disease via immunogenetics. J Autoimmun 2015;64:91-100. https://doi.org/10.1016/j.jaut.2015.07.013.
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491(7422):119-24. https://doi.org/10.1038/nature11582.
Yadav V, Varum F, Bravo R, Furrer E, Bojic D, Basit AW. Inflammatory bowel disease: Exploring gut pathophysiology for novel therapeutic targets. Transl Res 2016;176:38-68. https://doi.org/10.1016/j.trsl.2016.04.009.
Dudarewicz M, Baranska M, Rychlik-Sych M, Trzcinski R, Dziki A, Skretkowicz J. C3435T polymorphism of the ABCB1/MDR1 gene encoding P-glycoprotein in patients with inflammatory bowel disease in a Polish population. Pharmacol Rep 2012;64(2):343-50. https://doi.org/10.1016/S1734-1140(12)70774-0.
Brinar M, Cukovic-Cavka S, Bozina N, Ravic KG, Markos P, Ladic A, et al. MDR1 polymorphisms are associated with inflammatory bowel disease in a cohort of Croatian IBD patients. BMC Gastroenterol 2013;13:57. https://doi.org/10.1186/1471-230X-13-57.
Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis 2010;4(1):7-27. https://doi.org/10.1016/j.crohns.2009.12.003.
Farnood A, Naderi N, Moghaddam SJ, Noorinayer B, Firouzi F, Aghazadeh R, et al. The frequency of C3435T MDR1 gene polymorphism in Iranian patients with ulcerative colitis. Int J Colorectal Dis 2007;22(9):999-1003. https://doi.org/10.1007/s00384-007-0270-6.
Kurzawski M, Pawlik A, Górnik W, Drozdzik M. Frequency of common MDR1 gene variants in a Polish population. Pharmacol Rep 2006;58(1):35-40.
Fiedler T, Büning C, Reuter W, Pitre G, Gentz E, Schmidt HH, et al. Possible role of MDR1 two-locus genotypes for young-age onset ulcerative colitis but not Crohn's disease. Eur J Clin Pharmacol 2007;63(10):917-25. https://doi.org/10.1007/s00228-007-0334-0.
Schwab M, Schaeffeler E, Marx C, Fromm MF, Kaskas B, Metzler J, et al. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology 2003;124(1):26-33. https://doi.org/10.1053/gast.2003.50010.
Ho GT, Nimmo ER, Tenesa A, Fennell J, Drummond H, Mowat C, et al. Allelic variations of the multidrug resistance gene determine susceptibility and disease behavior in ulcerative colitis. Gastroenterology 2005;128(2):288-96. https://doi.org/10.1053/j.gastro.2004.11.019.
Onnie CM, Fisher SA, Pattni R, Sanderson J, Forbes A, Lewis CM, et al. Associations of allelic variants of the multidrug resistance gene (ABCB1 or MDR1) and inflammatory bowel disease and their effects on disease behavior: A case-control and meta-analysis study. Inflamm Bowel Dis 2006;12(4):263-71. https://doi.org/10.1097/01.MIB.0000209791.98866.ba.
Huebner C, Browning BL, Petermann I, Han DY, Philpott M, Barclay M, et al. Genetic analysis of MDR1 and inflammatory bowel disease reveals protective effect of heterozygous variants for ulcerative colitis. Inflamm Bowel Dis 2009;15(12):1784-93. https://doi.org/10.1002/ibd.21019.
Jazwinska-Tarnawska E, Jeskowiak I, Waszczuk E, Mulak A, Glowacka K, Hurkacz M, et al. Genetic polymorphism of ABCB1 gene (C3435T) in patients with inflammatory bowel diseases. Is there any gender dependency? Pharmacol Rep 2015;67(2):294-8. https://doi.org/10.1016/j.pharep.2014.09.014.
Oostenbrug LE, Dijkstra G, Nolte IM, van Dullemen HM, Oosterom E, Faber KN, et al. Absence of association between the multidrug resistance (MDR1) gene and inflammatory bowel disease. Scand J Gastroenterol 2006;41(10):1174-82. https://doi.org/10.1080/00365520600575746.
Ardizzone S, Maconi G, Bianchi V, Russo A, Colombo E, Cassinotti A, et al. Multidrug resistance 1 gene polymorphism and susceptibility to inflammatory bowel disease. Inflamm Bowel Dis 2007;13(5):516-23. https://doi.org/10.1002/ibd.20108.
Potocnik U, Ferkolj I, Glavac D, Dean M. Polymorphisms in multidrug resistance 1 (MDR1) gene are associated with refractory Crohn disease and ulcerative colitis. Genes Immun 2004;5(7):530-9. https://doi.org/10.1038/sj.gene.6364123.
Gazouli M, Zacharatos P, Gorgoulis V, Mantzaris G, Papalambros E, Ikonomopoulos J. The C3435T MDR1 gene polymorphism is not associated with susceptibility for ulcerative colitis in Greek population. Gastroenterology 2004;126(1):367-9. https://doi.org/10.1053/j.gastro.2003.08.044.
Fischer S, Lakatos PL; Hungarian IBD Study Group, Lakatos L, Kovacs A, Molnar T, et al. ATP-binding cassette transporter ABCG2 (BCRP) and ABCB1 (MDR1) variants are not associated with disease susceptibility, disease phenotype response to medical therapy or need for surgery in Hungarian patients with inflammatory bowel diseases. Scand J Gastroenterol 2007;42(6):726-33. https://doi.org/10.1080/00365520601101559.
Urcelay E, Mendoza JL, Martín MC, Mas A, Martínez A, Taxonera C, et al. MDR1 gene: Susceptibility in Spanish Crohn's disease and ulcerative colitis patients. Inflamm Bowel Dis 2006;12(1):33-7. https://doi.org/10.1097/01.MIB.0000194184.92671.78.
Lal S, Stempak JM, Law C, Elkadri AA, Steinhart AH, Silverberg MS. Association between the C3435T polymorphism of the MDR1 gene and Crohn's disease. Inflamm Bowel Dis 2006;12(10):1006-7. https://doi.org/10.1097/01.mib.0000228999.37090.e1.
Glas J, Török HP, Schiemann U, Folwaczny C. MDR1 gene polymorphism in ulcerative colitis. Gastroenterology 2004;126(1):367.
Croucher PJ, Mascheretti S, Foelsch UR, Hampe J, Schreiber S. Lack of association between the C3435T MDR1 gene polymorphism and inflammatory bowel disease in two independent Northern European populations. Gastroenterology 2003;125(6):1919-20. https://doi.org/10.1053/j.gastro.2003.05.016.
Brant SR, Panhuysen CI, Nicolae D, Reddy DM, Bonen DK, Karaliukas R, et al. MDR1 Ala893 polymorphism is associated with inflammatory bowel disease. Am J Hum Genet 2003;73(6):1282-92. https://doi.org/10.1086/379927.
Wang J, Guo X, Yu S, Zhang J, Song J, Ji M, et al. MDR1 C3435T polymorphism and inflammatory bowel disease risk: A meta-analysis. Mol Biol Rep 2014;41(4):2679-85. https://doi.org/10.1007/s11033-014-3127-4.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-07-04
Published 2017-11-20









