Adapted methods for scanning electron microscopy (SEM) in assessment of human sperm morphology
DOI:
https://doi.org/10.17305/bjbms.2017.2173Keywords:
Scanning electron microscope, SEM, preparation techniques, human sperm morphology, infertility, clinical practice, semen, CPD, critical point drying, HMDS, hexamethyldisilazaneAbstract
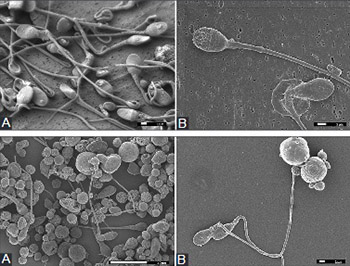
Infertility is a widespread problem, and in some cases, the routine basic semen analysis is not sufficient to detect the cause of male infertility. The use of the scanning electron microscope (SEM) could provide a detailed insight into spermatozoa morphology, but it requires specific sample preparation techniques. The purpose of this study was to select, adjust, and optimize a method for the preparation of spermatozoa samples prior to SEM analysis, and to establish the protocol required for its use in clinical practice. We examined sperm samples of 50 men. The samples were fixed with modified iso-osmolar aldehyde solution followed by osmium post-fixation. In the first method, dehydration of the cells and subsequent critical point drying (CPD) were performed on a coverslip. In the second method, the samples were dehydrated in centrifuge tubes; hexamethyldisilazane (HMDS) was used as a drying agent instead of CPD, and the samples were air-dried. The third procedure was based on a membrane filter. The samples were dehydrated and dried with HMDS in a Gooch crucible, continuously, without centrifugation or redispersion of the sample. Our results showed that the fixation with modified iso-osmolar aldehyde solution followed by osmium post-fixation, and combined with dehydration and CPD on a coverslip, is the most convenient procedure for SEM sample preparation. In the case of small-size samples or low sperm concentration, dehydration and drying with HMDS on the membrane filter enabled the best reliability, repeatability, and comparability of the results. The presented procedures are suitable for routine use, and they can be applied to confirm as well as to correct a diagnosis.
Citations
Downloads
References
World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Seme. 5th ed. Geneva: WHO Press; 2010.
Chemes EH, Rawe YV. Sperm pathology: A step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update 2003;9(5):405-28. https://doi.org/10.1093/humupd/dmg034.
Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci 2015;8(4):191-6.
https://doi.org/10.4103/0974-1208.170370.
Schirren C. Andrology. Origin and development of a special discipline in medicine. Reflection and view in the future. Andrologia 1985;17(2):117-25. https://doi.org/10.1111/j.1439-0272.1985.tb00970.x.
Moretti E, Collodel G. Electron microscopy in the study of human sperm pathologies. In: Mendez-Vilas A, editor. Current Microscopy Contributions to Advances in Science and Technology. Badajoz: Microscopy Book Series; 2014. p. 343-51.
Canillioglu YE, Senturk GE, Hurdag C. Examinations of sperm by light and electron microscopic levels: Friendly preparation techniques. In: Mendez-Vilas A, editor. Current Microscopy Contributions to Advances in Science and Technology. Badajoz: Microscopy Book Series; 2014. p. 662-8.
Visco V, Raffa S, Elia J, Delfino M, Imbrogno N, Torrisi MR, et al. Morphological sperm defects analyzed by light microscopy and transmission electron microscopy and their correlation with sperm motility. Int J Urol 2010;17(3):259-66.
https://doi.org/10.1111/j.1442-2042.2010.02451.x.
Vasan SS. Semen analysis and sperm function tests: How much to test? Indian J Urol 2011;27(1):41-8. https://doi.org/10.4103/0970-1591.78424.
Menkveld R. The basic semen analysis. In: Oehninger SC, Kruger TF, editors. Male Infertility Diagnosis and Treatment. London: Informa Healthcare; 2007. p. 141-70. https://doi.org/10.3109/9780203090626-12.
Nussdorfer P. Scanning Electron Microscopy Analysis in Assisted Reproduction Technology. M. Sc. Thesis. Ljubljana: Biotechnical Faculty; 2016.
Sathananthan AH. Ultrastructure of human gametes, fertilization and embryos in assisted reproduction: A personal survey. Micron 2013;44:1-20. https://doi.org/10.1016/j.micron.2012.05.002.
Sathananthan AH. Visual Atlas of Human Sperm Structure and Function for Assisted Reproductive Technology. Melbourne: La Trobe and Monash Universities; 1996.
Goodhew PJ, Humphreys J, Beanland R. Electron Microscopy and Analysis. New York: Taylor & Francis; 2000.
Watson LP, McKee AE, Merrell BR. Preparation of biological specimens for scanning electron microscopy. In: Murphy JA, Romans GM, editors. Preparation of Biological Specimens for Scanning Electron Microscopy. O’Hare: Scanning Electron Microscopy; 1980. p. 45-56.
Nowell JA, Pawley JB. Preparation of experimental animal tissue for SEM. In: Murphy JA, Romans GM, editors. Preparation of Biological Specimens for Scanning Electron Microscopy. O’Hare: Scanning Electron Microscopy; 1980. p. 1-19.
Pas M, Milacic R, Draslar K, Pollak N, Raspor P. Uptake of chromium(III) and chromium(VI) compounds in the yeast cell structure. Biometals 2004;17(1):25-33. https://doi.org/10.1023/A:1024437802914.
Murtey MD, Ramasamy P. Sample preparations for scanning electron microscopy. In: Janecek M, Kral R, editors. Physics, Optics and Lasers: Modern Electron Microscopy in Physical and Life Sciences. Penang: Intech; 2016. p. 163-85. https://doi.org/10.5772/61720.
Wisse E, Braet F, Duimel H, Vreuls C, Koek G, Olde Damink SW, et al. Fixation methods for electron microscopy of human and other liver. World J Gastroenterol 2010;16(23):2851-66. https://doi.org/10.3748/wjg.v16.i23.2851.
Romeis B. Mikroskopische technik. In: Böck P, editor. Neubearbeitete Auflage. München: Urban and Schwarzenberg; 1989. p. 150-5.
Andjelic S, Drašlar K, Hvala A, Hawlina M. Anterior lens epithelium in intumescent white cataracts-scanning and transmission electron microscopy study. Graefes Arch Clin Exp Ophthalmol 2016;254(2):269-76. https://doi.org/10.1007/s00417-015-3220-y.
Boyde A, Wood C. Preparation of animal tissues for surface-scanning electron microscopy. J Microsc 1969;90(3):221-49.
https://doi.org/10.1111/j.1365-2818.1969.tb00709.x.
Braet F, De Zanger R, Wisse E. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: A study on hepatic endothelial cells. J Microsc 1997;186(Pt 1):84-7. https://doi.org/10.1046/j.1365-2818.1997.1940755.x.
Shively S, Miller WR. The use of HMDS (hexamethyldisilazane) to replace critical point drying (CPD) in the preparation of tardigrade for SEM (scanning electron microscope) imaging. Trans Kans 2009;112(3-4):198-200. https://doi.org/10.1660/062.112.0407.
Behnke O, Zelander T. Preservation of intercellular substances by the cationic dye alcian blue in preparative procedures for electron microscopy. J Ultrastruct Res 1970;31(5-6):424-8. https://doi.org/10.1016/S0022-5320(70)90159-0.
Erlandsen SL, Kristich CJ, Dunny GM, Wells CL. High-resolution visualization of the microbial glycocalyx with low-voltage scanning electron microscopy: Dependence on cationic dyes. J Histochem Cytochem 2004;52(11):1427-35. https://doi.org/10.1369/jhc.4A6428.2004.
Mazia D, Schatten G, Sale W. Adhesion of cells to surfaces coated with polylysine. applications to electron microscopy. J Cell Biol 1975;66(1):198-200. https://doi.org/10.1083/jcb.66.1.198.
Jusman Y, Ng SC, Abu Osman NA. Investigation of CPD and HMDS sample preparation techniques for cervical cells in developing computer-aided screening system based on FE-SEM/EDX. SciWorld J 2014;2014:289817. https://doi.org/10.1155/2014/289817.
Kawai K, Kaneko K, Kawakami H, Narushima T, Yonezawa T. Simple pretreatment of non-conductive small hydrous bio-samples with choline-type ionic liquid and membrane filter for microsample mounting. Colloids Surf B Biointerfaces 2013;102:9-12. https://doi.org/10.1016/j.colsurfb.2012.08.019.
Todd RL, Kerr TJ. Scanning electron microscopy of microbial cells on membrane filters. Appl Microbiol 1972;23(6):1160-2.
Nussdorfer P, Virant-Klun I, Drašlar K, Tomaževič T, Meden-Vrtovec H. Scanning electron microscopy assay of sperm cells used in in vitro fertilization procedures. In: Ognjen M, editor. Proceedings of the 6th Multinational Congress on Microscopy-European Extension 2003. Pula: Croatian Society for Electron Microscopy; 2003. p. 248-9.
Zorn B, Ihan A, Kopitar AN, Kolbezen M, Sesek-Briski A, Meden-Vrtovec H. Changes in sperm apoptotic markers as related to seminal leukocytes and elastase. Reprod Biomed Online 2010;21(1):84-92. https://doi.org/10.1016/j.rbmo.2010.03.016.
Moretti E, Capitani S, Figura N, Pammolli A, Federico MG, Giannerini V, et al. The presence of bacteria species in semen and sperm quality. J Assist Reprod Genet 2009;26(1):47-56. https://doi.org/10.1007/s10815-008-9283-5.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-06-18
Published 2018-02-20









