The value of 18F-FDG PET/CT imaging in breast cancer staging
DOI:
https://doi.org/10.17305/bjbms.2017.2179Keywords:
Breast cancer, Breast cancer staging, 18F-FDG, 18F-FDG PET/CTAbstract
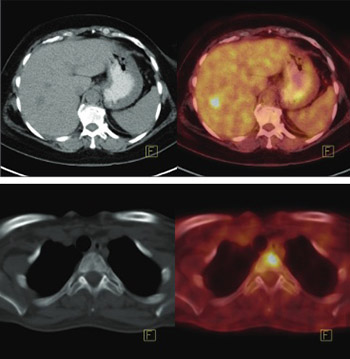
The National Comprehensive Cancer Network (NCCN) guidelines recommend assessment with positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed tomography (18F-FDG PET/CT) in staging of breast cancer, starting from the stage IIIA. Previously, PET/CT contributed to the accurate staging from the stage IIB. Our aim is to evaluate the contribution of 18F-FDG PET/CT in staging of breast cancer patients. A total of 234 patients were retrospectively evaluated. PET/CT was performed preoperatively in 114/234 and postoperatively in 120/234 patients. Initial staging was performed based on histopathological results in 125/234 and clinical results in 109/234 patients, according to the American Joint Committee on Cancer (AJCC) classification. All patients had a normal abdominal ultrasound and chest x-ray. Following PET/CT imaging, modification in the staging was performed in patients with the metastatic findings. In 42/234 (17.9%) patients hypermetabolic extra-axillary regional lymph nodes and in 65/234 patients (27.7%) distant metastatic involvement were detected with PET/CT. Modification in the staging was applied in 82/234 (35%) patients. Patient management was changed in 69/234 (29.4%) cases. The percentage of patients with upstaging, according to each stage, was as follows: IIA: 18.6%, IIB: 30%, IIIA: 46.3%, IIIB: 68.8%, and IIIC: 20.8%. In 43/43 patients, 99mTc-methylene diphosphonate (MDP) bone scan did not show additional bone metastasis. In 5/32 patients, metastatic involvement was detected with sentinel lymph node biopsy (SLNB), but preoperative PET/CT scan did not reveal hypermetabolic lymph nodes. Although our study was limited by the referral bias and lack of homogeneity in the referral group, PET/CT still significantly contributed to the accurate staging and management of our breast cancer patients, starting from the stage IIA.
Citations
Downloads
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59(4):225-49.
https://doi.org/10.3322/caac.20006.
Groheux D, Espié M, Giacchetti S, Hindié E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology 2013;266(2):388-405.
https://doi.org/10.1148/radiol.12110853.
Veronesi U, De Cicco C, Galimberti VE, Fernandez JR, Rotmensz N, Viale G, et al. A comparative study on the value of FDG-PET and sentinel node biopsy to identify occult axillary metastases. Ann Oncol 2007;18(3):473-8.
https://doi.org/10.1093/annonc/mdl425.
Evangelista L, Baretta Z, Vinante L, Cervino AR, Gregianin M, Ghiotto C, et al. Tumour markers and FDG PET/CT for prediction of disease relapse in patients with breast cancer. Eur J Nucl Med Mol Imaging 2011;38(2):293-301.
https://doi.org/10.1007/s00259-010-1626-7.
Aukema TS, Rutgers EJ, Vogel WV, Teertstra HJ, Oldenburg HS, Vrancken Peeters MT, et al. The role of FDG PET/CT in patients with locoregional breast cancer recurrence: A comparison to conventional imaging techniques. Eur J Surg Oncol 2010;36(4):387-92.
https://doi.org/10.1016/j.ejso.2009.11.009.
NCCN. Clinical Practice Guidelines in Oncology. Breast Cancer. Version 3; 2017. [cited 2017 Aug 10]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Groheux D, Giacchetti S, Espié M, Vercellino L, Hamy AS, Delord M, et al. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: A prospective study. J Nucl Med 2011;52(10):1526-34.
https://doi.org/10.2967/jnumed.111.093864.
Segaert I, Mottaghy F, Ceyssens S, De Wever W, Stroobants S, Van Ongeval C, et al. Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J 2010;16(6):617-24.
https://doi.org/10.1111/j.1524-4741.2010.00987.x.
Yararbas U, Argon AM, Yeniay L, Kapkac M. Problematic aspects of sentinel lymph node biopsy and its relation to previous excisional biopsy in breast cancer. Clin Nucl Med 2009;34(12):854-8.
https://doi.org/10.1097/RLU.0b013e3181becec2.
AJCC. Breast Cancer Staging. 8th ed. [cited 2017 Aug 15]. Available from: https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Breast%20Cancer%20Staging%20System.pdf.
Caldarella C, Treglia G, Giordano A. Diagnostic performance of dedicated positron emission mammography using fluorine-18-fluorodeoxyglucose in women with suspicious breast lesions: A meta-analysis. Clin Breast Cancer 2014;14(4):241-8.
https://doi.org/10.1016/j.clbc.2013.12.004.
Schilling K, Narayanan D, Kalinyak JE, The J, Velasquez MV, Kahn S, et al. Positron emission mammography in breast cancer presurgical planning: Comparisons with magnetic resonance imaging. Eur J Nucl Med Mol Imaging 2011;38(1):23-36.
https://doi.org/10.1007/s00259-010-1588-9.
Gil-Rendo A, Martínez-Regueira F, Zornoza G, García-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N. Association between [18F] fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg 2009;96(2):166-70.
https://doi.org/10.1002/bjs.6459.
Fujii T, Yajima R, Kurozumi S, Higuchi T, Obayashi S, Tokiniwa H, et al. Clinical significance of 18F-FDG-PET in invasive lobular carcinoma. Anticancer Res 2016;36(10):5481-5.
https://doi.org/10.21873/anticanres.11129.
Jung NY, Kim SH, Choi BB, Kim SH, Sung MS. Associations between the standardized uptake value of (18)F-FDG PET/CT and the prognostic factors of invasive lobular carcinoma: In comparison with invasive ductal carcinoma. World J Surg Oncol 2015;13:113.
https://doi.org/10.1186/s12957-015-0522-9.
Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat 2008;107(3):309-30.
https://doi.org/10.1007/s10549-007-9556-1.
Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): A phase 3 randomised controlled trial. Lancet Oncol 2013;14(4):297-305.
https://doi.org/10.1016/S1470-2045(13)70035-4.
Reimer T, Hartmann S, Stachs A, Gerber B. Local treatment of the axilla in early breast cancer: Concepts from the national surgical adjuvant breast and bowel project B-04 to the planned intergroup sentinel mamma trial. Breast Care (Basel) 2014;9(2):87-95.
https://doi.org/10.1159/000360411.
Manca G, Volterrani D, Mazzarri S, Duce V, Svirydenka A, Giuliano A, et al. Sentinel lymph node mapping in breast cancer: A critical reappraisal of the internal mammary chain issue. Q J Nucl Med Mol Imaging 2014;58(2):114-26.
Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol 1999;17(8):2334-40.
https://doi.org/10.1200/JCO.1999.17.8.2334.
Galasko C. The anatomy and pathways of bone metastases. In: Weiss L, Gilbert A, editors. Bone Metastases. Boston: G.K. Hall; 1981. p. 49-63.
Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer. Implications for management. Eur J Cancer 2000;36(4):476-82.
https://doi.org/10.1016/S0959-8049(99)00331-7.
Shie P, Cardarelli R, Brandon D, Erdman W, Abdulrahim N. Meta-analysis: Comparison of F-18 fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clin Nucl Med 2008;33(2):97-101.
https://doi.org/10.1097/RLU.0b013e31815f23b7.
Groheux D, Cochet A, Humbert O, Alberini JL, Hindié E, Mankoff D. 18F-FDG PET/CT for staging and restaging of breast cancer. J Nucl Med 2016;57(Suppl 1):17S-26S. DOI: 10.2967/jnumed.115.157859.
Riedl CC, Slobod E, Jochelson M, Morrow M, Goldman DA, Gonen M, et al. Retrospective analysis of 18F-FDG PET/CT for staging asymptomatic breast cancer patients younger than 40 years. J Nucl Med 2014;55(10):1578-83.
https://doi.org/10.2967/jnumed.114.143297.
Koolen BB, Vrancken Peeters MJ, Aukema TS, Vogel WV, Oldenburg HS, van der Hage JA, et al. 18F-FDG PET/CT as a staging procedure in primary stage II and III breast cancer: Comparison with conventional imaging techniques. Breast Cancer Res Treat 2012;131(1):117-26.
https://doi.org/10.1007/s10549-011-1767-9.
Cochet A, Dygai-Cochet I, Riedinger JM, Humbert O, Berriolo-Riedinger A, Toubeau M, et al. 18F-FDG PET/CT provides powerful prognostic stratification in the primary staging of large breast cancer when compared with conventional explorations. Eur J Nucl Med Mol Imaging 2014;41(3):428-37.
https://doi.org/10.1007/s00259-013-2595-4.
Groheux D, Hindié E, Delord M, Giacchetti S, Hamy AS, de Bazelaire C, et al. Prognostic impact of (18) FDG-PET-CT findings in clinical stage III and IIB breast cancer. J Natl Cancer Inst 2012;104(24):1879-87.
https://doi.org/10.1093/jnci/djs451.
Jain S, Fisher C, Smith P, Millis RR, Rubens RD. Patterns of metastatic breast cancer in relation to histological type. Eur J Cancer 1993;29A(15):2155-7.
https://doi.org/10.1016/0959-8049(93)90053-I.
He H, Gonzalez A, Robinson E, Yang WT. Distant metastatic disease manifestations in infiltrating lobular carcinoma of the breast. AJR Am J Roentgenol 2014;202(5):1140-8.
https://doi.org/10.2214/AJR.13.11156.
Ferlicot S, Vincent-Salomon A, Médioni J, Genin P, Rosty C, Sigal-Zafrani B, et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer 2004;40(3):336-41.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-07-24
Published 2018-02-20









