Oligoasthenoteratozoospermic (OAT) men display altered phospholipase C ζ (PLCζ) localization and a lower percentage of sperm cells expressing PLCζ and post-acrosomal sheath WW domain-binding protein (PAWP)
DOI:
https://doi.org/10.17305/bjbms.2017.2208Keywords:
Oligoasthenoteratozoospermia, OAT, phospholipase C ζ, PLCζ, post-acrosomal sheath WW domain-binding protein, PAWP, semen quality, sperm cellsAbstract
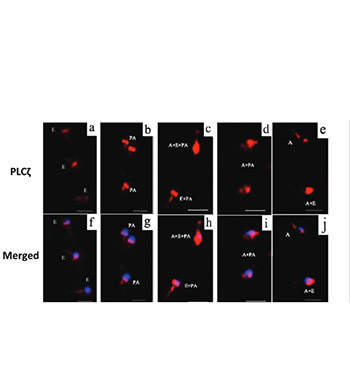
Oligoasthenoteratozoospermia (OAT) is demonstrated to be one of the most common causes of male subfertility. Phospholipase C ζ (PLCζ), a sperm-specific protein, is considered to be one of the sperm-borne oocyte activating factors (SOAFs), which play a vital role in fertilization. The post-acrosomal sheath WW domain-binding protein (PAWP) is another candidate for SOAF. The aim of this study was to compare the PLCζ localization patterns and percentage of PLCζ- and PAWP-positive sperm cells in patients with OAT and fertile men with normozoospermia. A total of 40 men included in this study were classified into two groups: OAT (n = 25) and control group (n = 15). Semen samples were collected and analyzed using conventional semen analysis according to the World Health Organization guidelines. The percentage of PLCζ- and PAWP-positive sperm cells and localization patterns of PLCζ were evaluated using immunofluorescence staining. The mean percentage of sperm cells expressing PAWP and PLCζ was significantly lower in OAT compared to control group (52.8 ± 4.2 vs. 76.8 ± 5 and 63.4 ± 3.5 vs. 86.7 ± 2.1, respectively). In addition, statistically significant differences were found with regard to the PLCζ localization patterns, including equatorial, acrosomal + equatorial, and equatorial + post-acrosomal pattern, between the two groups (p < 0.01). The present study showed a lower percentage of sperm cells expressing PLCζ and PAWP, as well as altered localization patterns of PLCζ in men with OAT. Given the role of PLCζ and PAWP in fertilization, as two major candidates for SOAFs, our findings indicate that PLCζ and PAWP impairments may be one of the possible etiologies of decreased fertility in OAT.
Citations
Downloads
References
Tournaye H, Krausz C, Oates RD. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol 2017;5(7):544-53.
https://doi.org/10.1016/S2213-8587(16)30040-7.
Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLCζ): Oocyte activation and clinical links to male factor infertility. Adv Biol Regul 2013;53(3):292-308. https://doi.org/10.1016/j.jbior.2013.07.005.
Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, et al. European association of urology guidelines on male infertility: The 2012 update. Eur Urol 2012;62(2):324-32. https://doi.org/10.1016/j.eururo.2012.04.048.
World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization Press; 2010.
Loutradi KE, Tarlatzis BC, Goulis DG, Zepiridis L, Pagou T, Chatziioannou E, et al. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J Assist Reprod Genet 2006;23(2):69-74. https://doi.org/10.1007/s10815-006-9022-8.
Zheng J, Lu Y, Qu X, Wang P, Zhao L, Gao M, et al. Correction: Decreased sperm motility retarded ICSI fertilization rate in severe oligozoospermia but good-quality embryo transfer had achieved the prospective clinical outcomes. PLoS One 2016:11(10):e0165684. https://doi.org/10.1371/journal.pone.0165684.
Lu YH, Gao HJ, Li BJ, Zheng YM, Ye YH, Qian YL, et al. Different sperm sources and parameters can influence intracytoplasmic sperm injection outcomes before embryo implantation. J Zhejiang Univ Sci B 2012;13(1):1-10. https://doi.org/10.1631/jzus.B1100216.
Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, Coward K. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update 2010;16(6):690-703. https://doi.org/10.1093/humupd/dmq018.
Park JH, Kim SK, Kim J, Kim JH, Chang JH, Jee BC, et al. Relationship between phospholipase C zeta immunoreactivity and DNA fragmentation and oxidation in human sperm. Obstet Gynecol Sci 2015;58(3):232-8. https://doi.org/10.5468/ogs.2015.58.3.232.
Yelumalai S, Yeste M, Jones C, Amdani SN, Kashir J, Mounce G, et al. Total levels, localization patterns, and proportions of sperm exhibiting phospholipase C zeta are significantly correlated with fertilization rates after intracytoplasmic sperm injection. Fertil Steril 2015;104(3):561-8.e4. https://doi.org/10.1016/j.fertnstert.2015.05.018.
Amdani SN, Yeste M, Jones C, Coward K. Phospholipase C zeta (PLCζ) and male infertility: Clinical update and topical developments. Adv Biol Regul 2016;61:58-67. https://doi.org/10.1016/j.jbior.2015.11.009.
Yu Y, Nomikos M, Theodoridou M, Nounesis G, Lai FA, Swann K. PLCζ causes Ca2+ oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P2. Mol Biol Cell 2012;23(2):371-80. https://doi.org/10.1091/mbc.E11-08-0687.
Swann K, Lai FA. PLCζ and the initiation of Ca2+ oscillations in fertilizing mammalian eggs. Cell Calcium 2013;53(1):55-62. https://doi.org/10.1016/j.ceca.2012.11.001.
Swann K, Lai FA. The sperm phospholipase C-ζ and Ca2+ signalling at fertilization in mammals. Biochem Soc Trans 2016;44(1):267-72. https://doi.org/10.1042/BST20150221.
Kashir J, Jones C, Mounce G, Ramadan WM, Lemmon B, Heindryckx B, et al. Variance in total levels of phospholipase C zeta (PLC-ζ) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil Steril 2013;99(1):107-17. https://doi.org/10.1016/j.fertnstert.2012.09.001.
Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest 2008;118(11):3671-81. https://doi.org/10.1172/JCI36942.
Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, et al. Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil Steril 2014;102(2):440-7. https://doi.org/10.1016/j.fertnstert.2014.05.003.
Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, et al. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J 2014;28(10):4434-40. https://doi.org/10.1096/fj.14-256495.
Aarabi M, Qin Z, Xu W, Mewburn J, Oko R. Sperm-borne protein, PAWP, initiates zygotic development in Xenopus laevis by eliciting intracellular calcium release. Mol Reprod Dev 2010;77(3):249-56. DOI: 10.1002/mrd.21140.
Grasa P, Coward K, Young C, Parrington J. The pattern of localization of the putative oocyte activation factor, phospholipase C zeta, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod 2008;23(11):2513-22. https://doi.org/10.1093/humrep/den280.
Nomikos M, Sanders JR, Kashir J, Sanusi R, Buntwal L, Love D, et al. Functional disparity between human PAWP and PLCζ in the generation of Ca2+ oscillations for oocyte activation. Mol Hum Reprod 2015;21(9):702-10. https://doi.org/10.1093/molehr/gav034.
Sutovsky P, Aarabi M, Miranda-Vizuete A, Oko R. Negative biomarker based male fertility evaluation: Sperm phenotypes associated with molecular-level anomalies. Asian J Androl 2015;17(4):554-60. https://doi.org/10.4103/1008-682X.153847.
Ramadan WM, Kashir J, Jones C, Coward K. Oocyte activation and phospholipase C zeta (PLCζ): Diagnostic and therapeutic implications for assisted reproductive technology. Cell Commun Signal 2012;10(1):12. https://doi.org/10.1186/1478-811X-10-12.
Tavalaee M, Parivar K, Nasr-Esfahani MH, Shahverdi A, Ghaedi K. A comparison of chromatin structure and PLCζ in sperms of subfertile oligoasthenoteratozoospermic and fertile men. J Shahrekord Univ Med Sci 2016;18(4):9-19.
Taylor SL, Yoon SY, Morshedi MS, Lacey DR, Jellerette T, Fissore RA, et al. Complete globozoospermia associated with PLCζ deficiency treated with calcium ionophore and ICSI results in pregnancy. Reprod Biomed Online 2010;20(4):559-64. https://doi.org/10.1016/j.rbmo.2009.12.024.
Kashir J, Sermondade N, Sifer C, Oo SL, Jones C, Mounce G, et al. Motile sperm organelle morphology evaluation-selected globozoospermic human sperm with an acrosomal bud exhibits novel patterns and higher levels of phospholipase C zeta. Hum Reprod 2012;27(11):3150-60. https://doi.org/10.1093/humrep/des312.
Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod 2009;24(10):2417-28. https://doi.org/10.1093/humrep/dep207.
Kamali-Dolat Abadi M, Tavalaee M, Shahverdi A, Nasr-Esfahani MH. Evaluation of PLCζ and PAWP expression in globozoospermic individuals. Cell J 2016;18(3):438-45. DOI: 10.22074/cellj.2016.4572.
Janghorban-Laricheh E, Ghazavi-Khorasgani N, Tavalaee M, Zohrabi D, Abbasi H, Nasr-Esfahani MH, et al. An association between sperm PLCζ levels and varicocele? J Assist Reprod Genet 2016;33(12):1649-55. https://doi.org/10.1007/s10815-016-0802-5.
Lee HC, Arny M, Grow D, Dumesic D, Fissore RA, Jellerette-Nolan T. Protein phospholipase C Zeta1 expression in patients with failed ICSI but with normal sperm parameters. J Assist Reprod Genet 2014;31(6):749-56. https://doi.org/10.1007/s10815-014-0229-9.
Chithiwala ZH, Lee HC, Hill DL, Jellerette-Nolan T, Fissore R, Grow D, et al. Phospholipase C-zeta deficiency as a cause for repetitive oocyte fertilization failure during ovarian stimulation for in vitro fertilization with ICSI: A case report. J Assist Reprod Genet 2015;32(9):1415-9. https://doi.org/10.1007/s10815-015-0531-1.
Durban M, Barragán M, Colodron M, Ferrer-Buitrago M, De Sutter P, Heindryckx B, et al. Erratum to: PLCζ disruption with complete fertilization failure in normozoospermia. J Assist Reprod Genet 2015;32(8):1295. https://doi.org/10.1007/s10815-015-0522-2.
Tavalaee M, Kiani-Esfahani A, Nasr-Esfahani MH. Relationship between potential sperm factors involved in oocyte activation and sperm DNA fragmentation with intra-cytoplasmic sperm injection clinical outcomes. Cell J 2017;18(4):588-96. DOI: 10.22074/cellj.2016.4725.
Ferrer-Vaquer A, Barragan M, Freour T, Vernaeve V, Vassena R. PLCζ sequence, protein levels, and distribution in human sperm do not correlate with semen characteristics and fertilization rates after ICSI. J Assist Reprod Genet 2016;33(6):747-56. https://doi.org/10.1007/s10815-016-0718-0.
Freour T, Barragan M, Ferrer-Vaquer A, Rodríguez A, Vassena R. WBP2NL/PAWP mRNA and protein expression in sperm cells are not related to semen parameters, fertilization rate, or reproductive outcome. J Assist Reprod Genet 2017;34(6):803-10.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-08-01
Published 2018-05-20









