The joint effect of the endothelin receptor B gene (EDNRB) polymorphism rs10507875 and nitric oxide synthase 3 gene (NOS3) polymorphism rs869109213 in Slovenian patients with type 2 diabetes mellitus and diabetic retinopathy
DOI:
https://doi.org/10.17305/bjbms.2017.2244Keywords:
Nitric oxide synthase 3, NOS3, endothelin receptor B, EDNRB, diabetic retinopathy, DR, type 2 diabetes mellitus, T2DM, polymorphism, genetic model of inheritanceAbstract
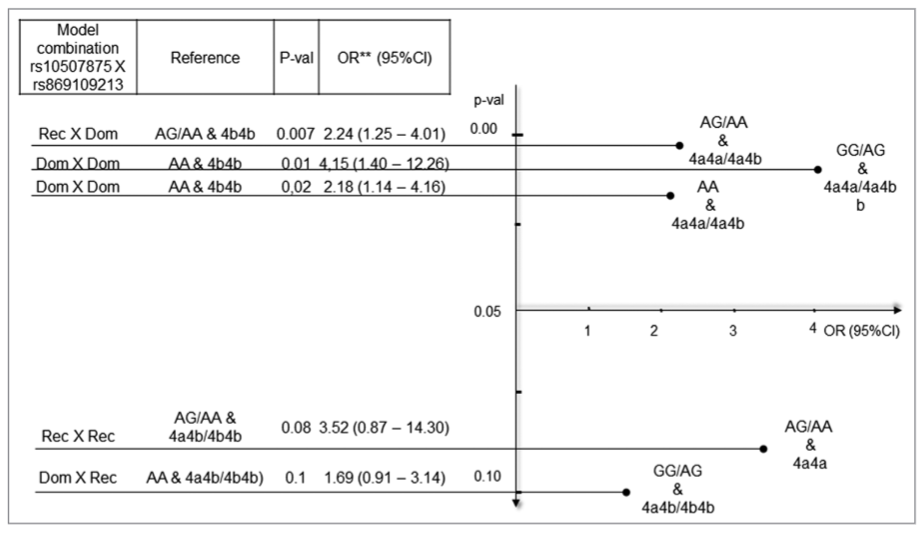
Increasing evidence suggests that endothelin and nitric oxide synthase genes and their products exert biological effects on the vasculature via the nitric oxide or endothelin pathway. The aim of the study was to evaluate the association of rs10507875 and rs869109213 (alone or in interaction) with diabetic retinopathy (DR) in subjects with type 2 diabetes mellitus (T2DM). We genotyped the single nucleotide polymorphism rs10507875 of the endothelin receptor B gene (EDNRB) and variable number tandem repeats rs869109213 of the nitric oxide synthase 3 gene (NOS3) in 270 Slovenian patients with DR and T2DM and 256 controls with T2DM without clinical signs of DR. The genotyping was performed using either real-time polymerase chain reaction (PCR) or standard PCR. We found a significant association between the genotypes of NOS3 rs869109213 polymorphism and the risk of DR in the co-dominant model (4a4b genotype; 1.99-fold increased risk [1.09-3.65]; 95% confidence interval [CI]; p = 0.02), co-dominant model (4a4a genotype; 4.16-fold increased risk [1.03-16.74]; 95% CI; p = 0.04), and dominant model (4a4a and 4a4b genotypes; 2.22-fold increased risk [1.26-3.92]; 95% CI; p = 0.01) compared to the 4b4b genotype. Moreover, the joint effect of the two polymorphisms on DR risk was greater than the individual effect of each polymorphism in the analyzed genetic models. Additionally, adjusted odds ratio showed an increased risk in dominant × dominant (4.15-fold [1.40-12.26]; 95% CI; p = 0.01) and recessive × dominant (2.24-fold [1.25-4.01]; 95% CI; p = 0.02) genotype combinations of the two polymorphisms. In conclusion, our results indicate that NOS3 rs869109213 polymorphism alone or in a combination with EDNRB rs10507875 polymorphism may be associated with DR in Slovenian patients with T2DM.
Citations
Downloads
References
Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, Inukai K, et al. A common polymorphism in the 5’-untranslated region of the VEGF gene is associated with diabetic retinopathy in Type 2 diabetes. Diabetes 2002;51(5):1635-9. https://doi.org/10.2337/diabetes.51.5.1635.
Lu Y, Shi Y, Ge Y, Yin J, Huang Z. Two polymorphisms (Rs699947, Rs2010963) in the VEGFA gene and diabetic retinopathy: An updated meta-analysis. J Diabetes Metab 2012;Suppl 3:6. https://doi.org/10.4172/2155-6156.S3-006.
Stitt AW, Lois N, Medina RJ, Adamson P, Curtis TM. Advances in our understanding of diabetic retinopathy. Clin Sci (Lond) 2013;125(1):1-17. https://doi.org/10.1042/CS20120588.
Huo L, Tao S, Jian-Mei G, Juan L, Xin-Min Y, Lin F. Genotyping analysis of a polymorphic G-954C of NOS2A in diabetic retinopathy with cystoid macular edema. Int J Ophthalmol 2008;1(2):95-8.
Yun JS, Ko SH, Kim JH, Moon KW, Park YM, Yoo KD, et al. Diabetic retinopathy and endothelial dysfunction in patients with Type 2 diabetes mellitus. Diabetes Metab J 2013;37(4):262-9. https://doi.org/10.4093/dmj.2013.37.4.262.
Warpeha KM, Chakravarthy U. Molecular genetics of microvascular disease in diabetic retinopathy. Eye (Lond) 2003;17(3):305-11. https://doi.org/10.1038/sj.eye.6700348.
Shah R. Endothelins in health and disease. Eur J Intern Med 2007;18(4):272-82. https://doi.org/10.1016/j.ejim.2007.04.002.
Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol 2007;47:731-59. https://doi.org/10.1146/annurev.pharmtox.47.120505.105134.
Vignon-Zellweger N, Heiden S, Miyauchi T, Emoto N. Endothelin and endothelin receptors in the renal and cardiovascular systems. Life Sci 2012;91(13-14):490-500. https://doi.org/10.1016/j.lfs.2012.03.026.
Mas M. A close look at the endothelium: Its role in the regulation of vasomotor tone. Eur Urol Suppl 2009;8(2):48-57. https://doi.org/10.1016/j.eursup.2008.10.011.
Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: New perspectives. Am J Physiol Regul Integr Comp Physiol 2011;300(6):R1288-95. https://doi.org/10.1152/ajpregu.00397.2010.
Stobdan T, Zhou D, Ao-Ieong E, Ortiz D, Ronen R, Hartley I, et al. Endothelin receptor B, a candidate gene from human studies at high altitude, improves cardiac tolerance to hypoxia in genetically engineered heterozygote mice. Proc Natl Acad Sci U S A 2015;112(33):10425-30. https://doi.org/10.1073/pnas.1507486112.
Prabhakar S, Noonan JP, Pääbo S, Rubin EM. Accelerated evolution of conserved noncoding sequences in humans. Science 2006;314(5800):786. https://doi.org/10.1126/science.1130738.
Oliveira-Paula GH, Lacchini R, Tanus-Santos JE. Endothelial nitric oxide synthase: From biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene 2016;575(2 Pt 3):584-99. https://doi.org/10.1016/j.gene.2015.09.061.
Kahn R. Expert commitee on the diagnosis and classification of diabetes mellitus. Follow up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;11(26):3160-7. https://doi.org/10.2337/diacare.26.11.3160.
Grading diabetic retinopathy from stereoscopic color fundus photographs-an extension of the modified Airlie house classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98(5 Suppl):786-806.
https://doi.org/10.1016/S0161-6420(13)38012-9.
Wang XL, Sim AS, Badenhop RF, McCredie RM, Wilcken DE. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nat Med 1996;2(1):41-5. https://doi.org/10.1038/nm0196-41.
Elston RC, Forthofer R. Testing for Hardy-Weinberg equilibrium small samples. Biometrics 1977;3(33):536-42. https://doi.org/10.2307/2529370.
Ma ZJ, Chen R, Ren HZ, Guo X, Guo J, Chen LM. Association between eNOS 4b/a polymorphism and the risk of diabetic retinopathy in Type 2 diabetes mellitus: A meta-analysis. J Diabetes Res 2014;2014:549747. http://dx.doi.org/10.1155/2014/549747.
Cilenšek I, Mankoc S, Globocnik Petrovic M, Petrovic D. The 4a/4a genotype of the VNTR polymorphism for endothelial nitric oxide synthase (eNOS) gene predicts risk for proliferative diabetic retinopathy in Slovenian patients (Caucasians) with Type 2 diabetes mellitus. Mol Biol Rep 2012;39(6):7061-7. https://doi.org/10.1007/s11033-012-1537-8.
Cheema BS, Kohli HS, Sharma R, Bhansali A, Khullar M. Endothelial nitric oxide synthase gene polymorphism and Type 2 diabetic retinopathy among Asian Indians. Acta Diabetol 2012;49(6):481-8. https://doi.org/10.1007/s00592-012-0437-7.
MacClellan LR, Howard TD, Cole JW, Stine OC, Giles WH, O’Connell JR, et al. Relation of candidate genes that encode for endothelial function to migraine and stroke: The Stroke Prevention in Young Women study. Stroke 2009;40(10):e550-7. https://doi.org/10.1161/STROKEAHA.109.557462.
Hein TW, Ren Y, Yuan Z, Xu W, Somvanshi S, Nagaoka T, et al. Functional and molecular characterization of the endothelin system in retinal arterioles. Invest Ophthalmol Vis Sci 2009;50(7):3329-36. https://doi.org/10.1167/iovs.08-3129.
Globočnik PM. The Influence of Genetical and Biochemical Factors for the Progression and Treatment of Diabetic Retinopathy in Type 2 Diabetes. Dissertation Thesis; 2006. p115.
Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide 2009;21(2):92-103. https://doi.org/10.1016/j.niox.2009.07.002.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2017 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2017-07-12
Published 2018-02-20









