The Kampo medicine Yokukansan (YKS) enhances nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells
DOI:
https://doi.org/10.17305/bjbms.2017.2248Keywords:
NGF, neurite outgrowth, Kampo, Yokukansan, Akt, ERK1/2, PC12 cells, YKS, TrkA inhibitor, nerve growth factorAbstract
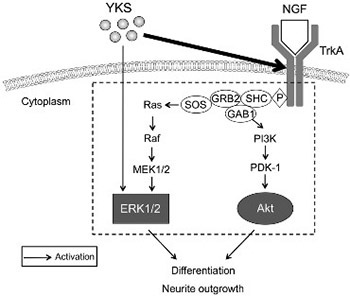
Accumulating evidence indicates that neurotrophic factor-like substances involved in the induction of neurotrophic factor synthesis may aid in the treatment of neurological disorders, such as Alzheimer’s disease. Yokukansan (YKS), a traditional Kampo medicine, has been used for the treatment of anxiety and mood disorders. In the present study, we aimed to identify the signaling pathways associated with YKS-mediated enhancement of nerve growth factor (NGF)-induced neurite extension in rat pheochromocytoma (PC12) cells. Akt and extracellular-regulated kinase 1/2 (ERK1/2) phosphorylation levels were assessed by western blot analysis, in the presence of YKS and following the treatment with TrkA inhibitor, K252a. YKS treatment (NGF+YKS 0.5 group) enhanced NGF-induced neurite outgrowth and phosphorylation/activation of Akt and ERK1/2 in PC12 cells. Moreover, YKS-induced effects were inhibited by the treatment with the TrkA receptor antagonist K252a (NGF+YKS 0.5+K252a group); no significant difference in neurite outgrowth was observed between K252a-treated (NGF+YKS 0.5+K252a group) and NGF-K252a-treated cells (NGF+K252a group). However, neurite outgrowth in K252a-treated cells (NGF+K252a and NGF+YKS 0.5+K252a group) reached only one-third of the level in NGF-treated cells (NGF group). NGF-mediated Akt phosphorylation increased by YKS was also inhibited by K252a treatment (NGF+YKS 0.5+K252a group), but no significant difference in ERK1/2 phosphorylation was observed between NGF-YKS-K252a- and NGF-treated cells (NGF group). Our results indicate that YKS treatment enhanced NGF-induced neurite outgrowth via induction of Akt and ERK1/2 phosphorylation, following the binding of NGF to the TrkA receptor. These findings may be useful in the development of novel therapeutic strategies for the treatment of Alzheimer’s disease.
Citations
Downloads
References
Mizukami K, Asada T, Kinoshita T, Tanaka K, Sonohara K, Nakai R, et al. A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int J Neuropsychopharmacol 2009;12(2):191-9. https://doi.org/10.1017/S146114570800970X.
Hatano T, Hattori N, Kawanabe T, Terayama Y, Suzuki N, Iwasaki Y, et al. An exploratory study of the efficacy and safety of yokukansan for neuropsychiatric symptoms in patients with Parkinson's disease. J Neural Transm (Vienna) 2014;121(3):275-81. https://doi.org/10.1007/s00702-013-1105-y.
Okamoto H, Iyo M, Ueda K, Han C, Hirasaki Y, Namiki T. Yokukan-san: A review of the evidence for use of this Kampo herbal formula in dementia and psychiatric conditions. Neuropsychiatr Dis Treat 2014;10:1727-42. https://doi.org/10.2147/NDT.S65257.
Iwasaki K, Satoh-Nakagawa T, Maruyama M, Monma Y, Nemoto M, Tomita N, et al. A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry 2005;66(2):248-52. https://doi.org/10.4088/JCP.v66n0214.
Sumiyoshi H, Mantani A, Nishiyama S, Fujiwaki S, Ohta S, Masuda Y, et al. Yokukansan treatment of chronic renal failure patients receiving hemodialysis, with behavioral and psychological symptoms of dementia: An open-label study. Am J Geriatr Psychiatry 2013;21(11):1082-5. https://doi.org/10.1016/j.jagp.2011.06.001.
Matsuda Y, Kishi T, Shibayama H, Iwata N. Yokukansan in the treatment of behavioral and psychological symptoms of dementia: A systematic review and meta-analysis of randomized controlled trials. Hum Psychopharmacol 2013;28(1):80-6. https://doi.org/10.1002/hup.2286.
Watanabe K, Matsuura K, Gao P, Hottenbacher L, Tokunaga H, Nishimura K, et al. Traditional Japanese Kampo medicine: Clinical research between modernity and traditional medicine-the state of research and methodological suggestions for the future. Evid Based Complement Alternat Med 2011;2011:513842. https://doi.org/10.1093/ecam/neq067.
Kawakami Z, Kanno H, Ueki T, Terawaki K, Tabuchi M, Ikarashi Y, et al. Neuroprotective effects of yokukansan, a traditional Japanese medicine, on glutamate-mediated excitotoxicity in cultured cells. Neuroscience 2009;159(4):1397-407. https://doi.org/10.1016/j.neuroscience.2009.02.004.
Hiratsuka T, Matsuzaki S, Miyata S, Kinoshita M, Kakehi K, Nishida S, et al. Yokukansan inhibits neuronal death during ER stress by regulating the unfolded protein response. PLoS One 2010;5:e13280. https://doi.org/10.1371/journal.pone.0013280.
Kubota K, Sano K, Shiraishi A, Beppu N, Nogami A, Uchida N, et al. Yokukansan, a traditional Japanese herbal medicine, promotes neurite outgrowth in PC12 cells through the activation of extracellular signal regulated kinase 1/2 and phosphatidylinositol 3-kinase/Akt. J Tradit Med 2013;30(3):102-13. https://doi.org/10.11339/jtm.30.102.
Ueki T, Mizoguchi K, Yamaguchi T, Nishi A, Ikarashi Y, Hattori T, et al. Yokukansan increases 5-HT1A receptors in the prefrontal cortex and enhances 5-HT1A receptor agonist-induced behavioral responses in socially isolated mice. Evid Based Complement Alternat Med 2015;2015:726471. https://doi.org/10.1155/2015/726471.
Sekiguchi K, Imamura S, Yamaguchi T, Tabuchi M, Kanno H, Terawaki K, et al. Effects of yokukansan and donepezil on learning disturbance and aggressiveness induced by intracerebroventricular injection of amyloid ß protein in mice. Phytother Res 2011;25(4):501-7. https://doi.org/10.1002/ptr.3287.
Ishida Y, Ebihara K, Tabuchi M, Imamura S, Sekiguchi K, Mizoguchi K, et al. Yokukansan, a traditional Japanese medicine, enhances the L-DOPA-induced rotational response in 6-hydroxydopamine-lesioned rats: Possible inhibition of COMT. Biol Pharm Bull 2016;39(1):104-13. https://doi.org/10.1248/bpb.b15-00691.
Egashira N, Nogami A, Iwasaki K, Ishibashi A, Uchida N, Takasaki K, et al. Yokukansan enhances pentobarbital-induced sleep in socially isolated mice: Possible involvement of GABA(A)-benzodiazepine receptor complex. J Pharmacol Sci 2011;116(3):316-20. https://doi.org/10.1254/jphs.11079SC.
Ikarashi Y, Mizoguchi K. Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol Ther 2016;166:84-95. https://doi.org/10.1016/j.pharmthera.2016.06.018.
Schäper C, Gläser S, Groneberg DA, Kunkel G, Ewert R, Noga O. Nerve growth factor synthesis in human vascular smooth muscle cells and its regulation by dexamethasone. Regul Pept 2009;157(1-3):3-7. https://doi.org/10.1016/j.regpep.2009.07.004.
Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 2001;24:1217-81. https://doi.org/10.1146/annurev.neuro.24.1.1217.
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell 2010;140(6):918-34. https://doi.org/10.1016/j.cell.2010.02.016.
Wigerius M, Asghar N, Melik W, Johansson M. Scribble controls NGF-mediated neurite outgrowth in PC12 cells. Eur J Cell Biol 2013;92(6-7):213-21. https://doi.org/10.1016/j.ejcb.2013.07.002.
Guroff G. PC12 cells as a model of neuronal differentiation. Cell Cult Neurosci 1985;1:245-72. https://doi.org/10.1007/978-1-4613-2473-7_8.
Yu CW, Chang PT, Hsin LW, Chern JW. Quinazolin-4-one derivatives as selective histone deacetylase-6 inhibitors for the treatment of Alzheimer’s disease. J Med Chem 2013;56(17):6775-91. https://doi.org/10.1021/jm400564j.
Rossi D, Pedrali A, Gaggeri R, Marra A, Pignataro L, Laurini E, et al. Chemical, pharmacological, and in vitro metabolic stability studies on enantiomerically pure RC-33 compounds: Promising neuroprotective agents acting as s1 receptor agonists. Chem Med Chem 2013;8(9):1514-27. https://doi.org/10.1002/cmdc.201300218.
Nishina A, Kimura H, Tsukagoshi H, Kozawa K, Koketsu M, Ninomiya M, et al. Neurite outgrowth of PC12 cells by 4'-O-ß-D-glucopyranosyl-3',4-dimethoxychalcone from Brassica rapa L. ‘hidabeni’ was enhanced by pretreatment with p38MAPK inhibitor. Neurochem Res 2013;38(11):2397-407. https://doi.org/10.1007/s11064-013-1152-7.
Zhao J, Cheng YY, Fan W, Yang CB, Ye SF, Cui W, et al. Botanical drug puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt signaling pathways in the neurite extension process. CNS Neurosci Ther 2015;21(1):61-70. https://doi.org/10.1111/cns.12334.
Kaplan DR, Stephens RM. Neurotrophin signal transduction by the Trk receptor. J Neurobiol 1994;25(11):1404-17. https://doi.org/10.1002/neu.480251108.
Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 1995;267(5206):2003-6. https://doi.org/10.1126/science.7701324.
Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci 2000;20(15):5671-8.
Mnich K, Carleton LA, Kavanagh ET, Doyle KM, Samali A, Gorman AM. Nerve growth factor-mediated inhibition of apoptosis post-caspase activation is due to removal of active caspase-3 in a lysosome-dependent manner. Cell Death Dis 2014;5:e1202. https://doi.org/10.1038/cddis.2014.173.
Ma K, Yan N, Huang Y, Cao G, Deng J, Deng Y. Effects of nerve growth factor on nerve regeneration after corneal nerve damage. Int J Clin Exp Med 2014;7(11):4584-9.
Xie Y, Ye L, Zhang X, Cui W, Lou J, Nagai T, et al. Transport of nerve growth factor encapsulated into liposomes across the blood-brain barrier: In vitro and in vivo studies. J Control Release 2005;105(1-2):106-19. https://doi.org/10.1016/j.jconrel.2005.03.005.
Tuszynski MH, Yang JH, Barba D, U HS, Bakay RA, Pay MM, et al. Nerve growth factor gene therapy: Activation of neuronal responses in Alzheimer disease. JAMA Neurol 2015;72(10):1139-47. https://doi.org/10.1001/jamaneurol.2015.1807.
Sobczak M, Chumak V, Pomorski P, Wojtera E, Majewski L, Nowak J, et al. Interaction of myosin VI and its binding partner DOCK7 plays an important role in NGF-stimulated protrusion formation in PC12 cells. Biochim Biophys Acta 2016;1863(7 Pt A):1589-600. https://doi.org/10.1016/j.bbamcr.2016.03.020.
Kordower JH, Mufson EJ. NGF receptor (p75)-immunoreactivity in the developing primate basal ganglia. J Comp Neurol 1993;327(3):359-75. https://doi.org/10.1002/cne.903270305.
Loy R, Lachyankar MB, Condon PJ, Poluha DK, Ross AH. Retrograde axonal transport and lesion-induced upregulation of the TrkA high-affinity NGF receptor. Exp Neurol 1994;130(2):377-86. https://doi.org/10.1006/exnr.1994.1217.
Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol 1985;101(5 Pt 1):1799-807. https://doi.org/10.1083/jcb.101.5.1799.
Tian X, Yue R, Zeng H, Li H, Shan L, He W, et al. Distinctive effect on nerve growth factor-induced PC12 cell neurite outgrowth by two unique neolignan enantiomers from Illicium merrillianum. Sci Rep 2015;5:16982. https://doi.org/10.1038/srep16982.
Terada K, Kojima Y, Watanabe T, Izumo N, Chiba K, Karube Y. Inhibition of nerve growth factor-induced neurite outgrowth from PC12 cells by dexamethasone: Signaling pathways through the glucocorticoid receptor and phosphorylated Akt and ERK1/2. PloS One 2014;9(3):e93223. https://doi.org/10.1371/journal.pone.0093223.
Hefti F, Armanini MP, Beck KD, Caras IW, Chen KS, Godowski PJ, et al. Development of neurotrophic factor therapy for Alzheimer’s disease. Ciba Found Symp 1996;196:54-63.
Webster NJ, Pirrung MC. Small molecule activators of the Trk receptors for neuroprotection. BMC Neurosci 2008;9(Suppl 2):S1.
https://doi.org/10.1186/1471-2202-9-S2-S1.
Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: Relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol 2002;68(3):209-45.
https://doi.org/10.1016/S0301-0082(02)00079-5.
Hashimoto S. K-252a, a potent protein kinase inhibitor, blocks nerve growth factor-induced neurite outgrowth and changes in the phosphorylation of proteins in PC12h cells. J Cell Biol 1988;107(4):1531-9. https://doi.org/10.1083/jcb.107.4.1531.
Das KP, Freudenrich TM, Mundy WR. Assessment of PC12 cell differentiation and neurite growth: A comparison of morphological and neurochemical measures. Neurotoxicol Teratol 2004;26(3):397-406. https://doi.org/10.1016/j.ntt.2004.02.006.
Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, et al. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem 1994;269(29):18961-7.
Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell 1995;80(2):179-85. https://doi.org/10.1016/0092-8674(95)90401-8.
Chambard JC, Lefloch R, Pouysségur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta 2007;1773(8):1299-310. https://doi.org/10.1016/j.bbamcr.2006.11.010.
Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell 2007;129(7):1261-74. https://doi.org/10.1016/j.cell.2007.06.009.
Kim Y, Seger R, Suresh Babu CV, Hwang SY, Yoo YS. A positive role of the PI3-K/Akt signaling pathway in PC12 cell differentiation. Mol Cells 2004;18(3):353-9.
Cerezo-Guisado MI, García-Román N, García-Marín LJ, Alvarez-Barrientos A, Bragado MJ, Lorenzo MJ. Lovastatin inhibits the extracellular-signal-regulated kinase pathway in immortalized rat brain neuroblasts. Biochem J 2007;401(1):175-83. https://doi.org/10.1042/BJ20060731.
Price RD, Yamaji T, Matsuoka N. FK506 potentiates NGF-induced neurite outgrowth via the Ras/Raf/MAP kinase pathway. Br J Pharmacol 2003;140(5):825-9. https://doi.org/10.1038/sj.bjp.0705522.
Terawaki K, Ikarashi Y, Sekiguchi K, Nakai Y, Kase Y. Partial agonistic effect of yokukansan on human recombinant serotonin 1A receptors expressed in the membranes of Chinese hamster ovary cells. J Ethnopharmacol 2010;127(2):306-12. https://doi.org/10.1016/j.jep.2009.11.003.
Nishi A, Yamaguchi T, Sekiguchi K, Imamura S, Tabuchi M, Kanno H, et al. Geissoschizine methyl ether, an alkaloid in Uncaria hook, is a potent serotonin (1)A receptor agonist and candidate for amelioration of aggressiveness and sociality by yokukansan. Neuroscience 2012;207:124-36. https://doi.org/10.1016/j.neuroscience.2012.01.037.
Imamura S, Tabuchi M, Kushida H, Nishi A, Kanno H, Yamaguchi T, et al. The blood-brain barrier permeability of geissoschizine methyl ether in Uncaria hook, a galenical constituent of the traditional Japanese medicine yokukansan. Cell Mol Neurobiol 2011;31(5):787-93. https://doi.org/10.1007/s10571-011-9676-3.
Tabuchi M, Imamura S, Kawakami Z, Ikarashi Y, Kase Y. The blood-brain barrier permeability of 18ß-glycyrrhetinic acid, a major metabolite of glycyrrhizin in Glycyrrhiza root, a constituent of the traditional Japanese medicine yokukansan. Cell Mol Neurobiol 2012;32(7):1139-46. https://doi.org/10.1007/s10571-012-9839-x.
Kushida H, Fukutake M, Tabuchi M, Katsuhara T, Nishimura H, Ikarashi Y, et al. Simultaneous quantitative analyses of indole and oxindole alkaloids of Uncaria Hook in rat plasma and brain after oral administration of the traditional Japanese medicine Yokukansan using high-performance liquid chromatography with tandem mass spectrometry. Biomed Chromatogr 2013;27(12):1647-56. https://doi.org/10.1002/bmc.2974.
Varsano T, Dong MQ, Niesman I, Gacula H, Lou X, Ma T, et al. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol Cell Biol 2006;26(3):8942-52. https://doi.org/10.1128/MCB.00305-06.
Lu J, Wu DM, Hu B, Zheng YL, Zhang ZF, Wang YJ. NGF-dependent activation of TrkA pathway: A mechanism for the neuroprotective effect of troxerutin in D-galactose-treated mice. Brain Pathol 2010;20(5):952-65.
Downloads
Additional Files
Published
How to Cite
Accepted 2017-08-21
Published 2018-08-01









