Comprehensive analysis of patients with neuromyelitis optica spectrum disorder (NMOSD) combined with chronic hepatitis B (CHB) infection and seropositive for anti-aquaporin-4 antibody
DOI:
https://doi.org/10.17305/bjbms.2017.2255Keywords:
Aquaporin-4, AQP4, neuromyelitis optica spectrum disorder, NMOSD, hepatitis B, HBV, CHB, liver functionAbstract
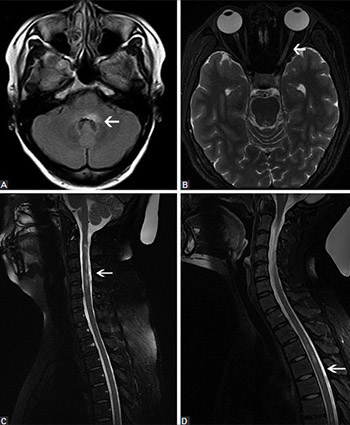
Previous research indicated the association between hepatitis B virus (HBV) infection/vaccination and the onset of demyelinating diseases. However, most of these studies were single case reports, and comprehensive data are still scarce. Here we present a comprehensive analysis of 10 patients with neuromyelitis optica spectrum disorder (NMOSD) combined with chronic hepatitis B (CHB) infection and seropositive for anti-aquaporin-4 antibody (AQP4-Ab). Demographic, clinical, laboratory, neuroimaging, outcome, and follow-up data of the 10 patients were retrospectively analyzed. The median age at the onset of NMOSD was 35 years (range 25-43). Nine patients were female (90%). All patients were positive for HBsAg and had been diagnosed with CHB earlier than with NMOSD. One patient had an autoimmune disease. All patients had normal thyroid function. Paresthesia and visual impairment were the most common clinical symptoms. The cerebrospinal fluid (CSF) parameters (protein and glucose) were normal in 10 cases, whereas slightly higher CSF white blood cell count was detected in 3 patients. The brain and spinal cord magnetic resonance imaging findings were abnormal in 8 patients. All patients were treated with hormone and immunosuppressive therapy, and anti-HBV agents. Patients with detectable serum HBV DNA were more prone to liver damage after receiving high doses of corticosteroids. In 8 patients, the symptoms improved before they were discharged. Two patients with optic neuritis (ON) maintained the symptoms. A month later, 1/8 patient had recurrence of symptoms, and one ON patient progressed to NMO. Overall, the characteristics of NMOSD patients with CHB and seropositive for AQP4-Ab are usually nonspecific. Abnormal liver function test results in NMOSD patients should be a warning of possible CHB infection, and the treatment should be modified accordingly.
Citations
Downloads
References
Ferro JM, Oliveira S. Neurologic manifestations of gastrointestinal and liver diseases. Curr Neurol Neurosci Rep 2014;14(10):487. https://doi.org/10.1007/s11910-014-0487-z.
Hepatitis B vaccines. WHO position paper. Weekly epidemiological record. 2009;84(40): 405-20.
Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27(47):6550-7. https://doi.org/10.1016/j.vaccine.2009.08.048.
Cacoub P, Terrier B. Hepatitis B-related autoimmune manifestations. Rheum Dis Clin North Am 2009;35(1):125-37. https://doi.org/10.1016/j.rdc.2009.03.006.
Lazibat I, Brinar V. Acute disseminated encephalomyelitis associated with hepatitis B virus reinfection--consequence or coincidence? Clin Neurol Neurosurg 2013;115(Suppl 1):S35-7. https://doi.org/10.1016/j.clineuro.2013.09.018.
Zhao S, Chen T, Peng C, Zhou H, Li H, Huang D, et al. The putative acceleration of optic neuritis when combined with chronic hepatitis B. J Neurol Sci 2015;358(1-2):207-12. https://doi.org/10.1016/j.jns.2015.08.1538.
Immunization Safety Review: Hepatitis B Vaccine and Demyelinating Neurological Disorders. Institute of Medicine (US) Immunization Safety Review Committee, Stratton K, Almario D, McCormick MC, editors. Washington (DC): National Academies Press (US); 2002.
Trevisani F, Gattinara GC, Caraceni P, Bernardi M, Albertoni F, D'Alessandro R, et al. Transverse myelitis following hepatitis B vaccination. J Hepatol 1993;19(2):317-8. https://doi.org/10.1016/S0168-8278(05)80589-6.
Jiang ZC, Liu ZH, Li HY, Wei SH. Optic neuritis combined with hepatitis B and neurosyphilis. Chin Med J (Engl) 2013;126(18):3580-1.
Heekin R, Gandhy C, Robertson D. Seronegative neuromyelitis optica spectrum disorder following exposure to hepatitis B vaccination. Case Rep Neurol 2015;7(1):78-83. https://doi.org/10.1159/000381826.
Sarri G, Westby M, Bermingham S, Hill-Cawthorne G, Thomas H, Guideline Development Group. Diagnosis and management of chronic hepatitis B in children, young people, and adults: Summary of NICE guidance. BMJ 2013;346:f3893. https://doi.org/10.1136/bmj.f3893.
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66(10):1485-9. https://doi.org/10.1212/01.wnl.0000216139.44259.74.
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85(2):177-89. https://doi.org/10.1212/WNL.0000000000001729.
Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: Acute, preventive, and symptomatic. Curr Treat Options Neurol 2016;18(1):2. https://doi.org/10.1007/s11940-015-0387-9.
Kimbrough DJ, Fujihara K, Jacob A, Lana-Peixoto MA, Leite MI, Levy M, et al. Treatment of neuromyelitis optica: Review and recommendations. Mult Scler Relat Disord 2012;1(4):180-7. https://doi.org/10.1016/j.msard.2012.06.002.
Trebst C, Jarius S, Berthele A, Paul F, Schippling S, Wildemann B, et al. Update on the diagnosis and treatment of neuromyelitis optica: Recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol 2014;261(1):1-16.
https://doi.org/10.1007/s00415-013-7169-7.
Qiu W, Kermode AG, Li R, Dai Y, Wang Y, Wang J, et al. Azathioprine plus corticosteroid treatment in Chinese patients with neuromyelitis optica. J Clin Neurosci 2015;22(7):1178-82. https://doi.org/10.1016/j.jocn.2015.01.028.
DeStefano F, Verstraeten T, Jackson LA, Okoro CA, Benson P, Black SB, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol 2003;60(4):504-9. https://doi.org/10.1001/archneur.60.4.504.
Bogdanos DP, Smith H, Ma Y, Baum H, Mieli-Vergani G, Vergani D. A study of molecular mimicry and immunological cross-reactivity between hepatitis B surface antigen and myelin mimics. Clin Dev Immunol 2005;12(3):217-24. https://doi.org/10.1080/17402520500285247.
Mastronardi FG, Moscarello MA. Molecules affecting myelin stability: A novel hypothesis regarding the pathogenesis of multiple sclerosis. J Neurosci Res 2005;80(3):301-8. https://doi.org/10.1002/jnr.20420.
Yang L, Tan D, Piao H. Myelin basic protein citrullination in multiple sclerosis: A potential therapeutic target for the pathology. Neurochem Res 2016;41(8):1845-56. https://doi.org/10.1007/s11064-016-1920-2.
Spadaro M, Gerdes LA, Krumbholz M, Ertl-Wagner B, Thaler FS, Schuh E, et al. Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016;3(5):e257.
http://dx.doi.org/10.1212/NXI.0000000000000257.
Thulasirajah S, Pohl D, Davila-Acosta J, Venkateswaran S. Myelin oligodendrocyte glycoprotein-associated pediatric central nervous system demyelination: Clinical course, neuroimaging findings, and response to therapy. Neuropediatrics 2016;47(4):245-52. https://doi.org/10.1055/s-0036-1583184.
Kim SM, Woodhall MR, Kim JS, Kim SJ, Park KS, Vincent A, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm 2015;2(6):e163. http://dx.doi.org/10.1212/NXI.0000000000000163.
Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016;13(1):280.
https://doi.org/10.1186/s12974-016-0718-0.
Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation 2016;13(1):279. https://doi.org/10.1186/s12974-016-0717-1.
Pohl M, Fischer MT, Mader S, Schanda K, Kitic M, Sharma R, et al. Pathogenic T cell responses against aquaporin 4. Acta Neuropathol 2011;122(1):21-34. DOI: 10.1007/s00401-011-0824-0.
Mitsdoerffer M, Kuchroo V, Korn T. Immunology of neuromyelitis optica: A T cell-B cell collaboration. Ann N Y Acad Sci 2013;1283:57-66. DOI: 10.1111/nyas.12118.
Vaknin-Dembinsky A, Brill L, Kassis I, Petrou P, Ovadia H, Ben-Hur T, et al. T-cell reactivity against AQP4 in neuromyelitis optica. Neurology 2012;79(9):945-6. https://doi.org/10.1212/WNL.0b013e318266fc2b.
Vaknin-Dembinsky A, Brill L, Kassis I, Petrou P, Ovadia H, Ben-Hur T, et al. T-cell responses to distinct AQP4 peptides in patients with neuromyelitis optica (NMO). Mult Scler Relat Disord 2016;6:28-36. https://doi.org/10.1016/j.msard.2015.12.004.
Melamed E, Levy M, Waters PJ, Sato DK, Bennett JL, John GR, et al. Update on biomarkers in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm 2015;2(4):e134. http://dx.doi.org/10.1212/NXI.0000000000000134.
Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: What is the evidence? Lancet 2003;362(9396):1659-66. https://doi.org/10.1016/S0140-6736(03)14802-7.
Piaggio E, Ben Younes A, Desbois S, Gout O, Tourbah A, Lyon-Caen O, et al. Hepatitis B vaccination and central nervous system demyelination: An immunological approach. J Autoimmun 2005;24(1):33-7. https://doi.org/10.1016/j.jaut.2004.11.007.
Biswas A, Mukherjee A. Therapy of NMO spectrum disorders. Ann Indian Acad Neurol 2015;18(Suppl 1):S16-23. https://doi.org/10.4103/0972-2327.164818.
Sato DK, Lana-Peixoto MA, Fujihara K, de Seze J. Clinical spectrum and treatment of neuromyelitis optica spectrum disorders: Evolution and current status. Brain Pathol 2013;23(6):647-60. https://doi.org/10.1111/bpa.12087.
Stellmann JP, Krumbholz M, Friede T, Gahlen A, Borisow N, Fischer K, et al. Immunotherapies in neuromyelitis optica spectrum disorder: Efficacy and predictors of response. J Neurol Neurosurg Psychiatry 2017;88(8):639-47.
https://doi.org/10.1136/jnnp-2017-315603.
Marino M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C. Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves' ophthalmopathy. Thyroid 2004;14(5):403-6. https://doi.org/10.1089/105072504774193276.
Eguchi H, Tani J, Hirao S, Tsuruta M, Tokubuchi I, Yamada K, et al. Liver dysfunction associated with intravenous methylprednisolone pulse therapy in patients with Graves' orbitopathy. Int J Endocrinol 2015;2015:835979. http://dx.doi.org/10.1155/2015/835979.
Costanzi C, Matiello M, Lucchinetti CF, Weinshenker BG, Pittock SJ, Mandrekar J, et al. Azathioprine: Tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology 2011;77(7):659-66. https://doi.org/10.1212/WNL.0b013e31822a2780.
Liu YP, Xu HQ, Li M, Yang X, Yu S, Fu WL, et al. Association between thiopurine s-methyltransferase polymorphisms and azathioprine-induced adverse drug reactions in patients with autoimmune diseases: A meta-analysis. PloS One 2015;10(12):e0144234. https://doi.org/10.1371/journal.pone.0144234.
Mok MY, Ng WL, Yuen MF, Wong RW, Lau CS. Safety of disease modifying anti-rheumatic agents in rheumatoid arthritis patients with chronic viral hepatitis. Clin Exp Rheumatol 2000;18(3):363-8.
Downloads
Additional Files
Published
How to Cite
Accepted 2017-08-31
Published 2018-02-20









