Vitamin D status, serum lipid concentrations, and vitamin D receptor (VDR) gene polymorphisms in Familial Mediterranean fever
DOI:
https://doi.org/10.17305/bjbms.2017.2259Keywords:
25(OH)D3, FMF, Familial Mediterranean fever, serum lipids, VDR polymorphismsAbstract
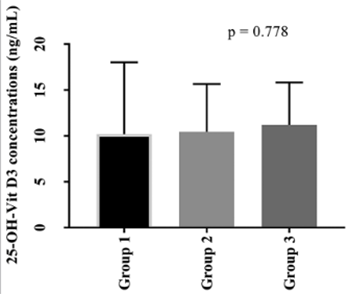
Vitamin D (VitD) is critical for the regulation of inflammatory processes, and VitD deficiency has been linked to several chronic inflammatory disorders. We aimed to investigate the concentrations of serum 25(OH)D3, lipid parameters, and three known VDR polymorphisms (BsmI, FokI, and TaqI) in patients with Familial Mediterranean fever (FMF), an autosomal recessive autoinflammatory disease. The study included 123 FMF patients and 105 controls. Seventy patients had no attack (group 1), 30 had 1-2 attacks (group 2), and 23 had 3 or more attacks (group 3) within last three months. Serum 25(OH)D3 concentrations were determined using liquid chromatography–tandem mass spectrometry. BsmI, FokI, and TaqI polymorphisms were analyzed by a competitive allele specific polymerase chain reaction assay (KASPar). Serum lipid parameters were measured with enzymatic colorimetric methods. 25(OH)D3 concentrations were lower in FMF patients compared to controls (p < 0.001). No difference was observed in 25(OH)D3 concentration between groups 1, 2, and 3. The distributions of FokI and TaqI genotypes were not significantly different between FMF patients and controls. There was a significant difference in the distribution of AA BsmI genotype between male FMF patients and male controls. Increased concentrations of triglycerides (p = 0.012) and decreased concentrations of high-density lipoprotein cholesterol [HDL-C] (p = 0.006) were found in FMF patients compared to controls. Although lower 25(OH)D3 concentrations were observed in FMF patients versus controls, no association was determined between FMF attack frequency and 25(OH)D3 concentrations. We showed that the AA genotype of BsmI polymorphism is associated with FMF in males but not in females. The effects of decreased HDL-C and increased triglyceride concentrations on cardiovascular events in FMF patients should be further investigated.
Citations
Downloads
References
Shohat M, Halpern GJ. Familial Mediterranean fever - A review. Genet Med 2011;13(6):487-98. https://doi.org/10.1097/GIM.0b013e3182060456.
Zadeh N, Getzug T, Grody WW. Diagnosis and management of familial Mediterranean fever: Integrating medical genetics in a dedicated interdisciplinary clinic. Genet Med 2011;13(3):263-9. https://doi.org/10.1097/GIM.0b013e31820e27b1.
Dogan HO, Koca Y, Erden G, Karaaslan Y, Bozat H. Evaluating MEFV mutation frequency in Turkish familial Mediterranean fever suspected patients and gender correlation: A retrospective study. Mol Biol Rep 2012;39(5):6193-6.
https://doi.org/10.1007/s11033-011-1437-3.
Sugiura T, Kawaguchi Y, Fujikawa S, Hirano Y, Igarashi T, Kawamoto M, et al. Familial Mediterranean fever in three Japanese patients, and a comparison of the frequency of MEFV gene mutations in Japanese and Mediterranean populations. Mod Rheumatol 2008;18(1):57-9. https://doi.org/10.3109/s10165-007-0003-2.
Ben-Chetrit E, Touitou I. Familial Mediterranean fever in the world. Arthritis Rheum 2009;61(10):1447-53. https://doi.org/10.1002/art.24458.
Martinon F, Tschopp J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004;117(5):561-74. https://doi.org/10.1016/j.cell.2004.05.004.
Özen S, Batu ED, Demir S. Familial Mediterranean fever: Recent developments in pathogenesis and new recommendations for management. Front Immunol 2017;8:253. https://doi.org/10.3389/fimmu.2017.00253.
Henderson C, Goldbach-Mansky R. Monogenic autoinflammatory diseases: New insights into clinical aspects and pathogenesis. Curr Opin Rheumatol 2010;22(5):567-78. https://doi.org/10.1097/BOR.0b013e32833ceff4.
Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: Beyond the inflammasomes. PLoS Pathog 2010;6(2):e1000661. https://doi.org/10.1371/journal.ppat.1000661.
Wöbke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol 2014;5:244. https://doi.org/10.3389/fphys.2014.00244.
Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, et al. Cloning and expression of full-length cDNA encoding human Vitamin D receptor. Proc Natl Acad Sci U S A 1988;85(10):3294-8. https://doi.org/10.1073/pnas.85.10.3294.
Uitterlinden AG, Fang Y, van Meurs JB, van Leeuwen H, Pols HA. Vitamin D receptor gene polymorphisms in relation to Vitamin D related disease states. J Steroid Biochem Mol Biol 2004;89-90(1-5):187-93. https://doi.org/10.1016/j.jsbmb.2004.03.083.
Keles N, Aksu F, Aciksari G, Yılmaz Y, Demircioğlu K, Köstek O, et al. Is triglyceride/HDL ratio a reliable screening test for assessment of atherosclerotic risk in patients with chronic inflammatory disease? North Clin Istanbul 2016;3(1):39-45. DOI: 10.14744/nci.2016.52824.
Acay A, Ulu MS, Ahsen A, Ozkececi G, Demir K, Ozuguz U, et al. Atherogenic index as a predictor of atherosclerosis in subjects with familial Mediterranean fever. Medicina (Kaunas) 2014;50(3):329-33. https://doi.org/10.1016/j.medici.2014.11.009.
Candan Z, Akdogan A, Karadag Ö, Kalyoncu U, Sahin A, Bilgen S, et al. Serum lipid changes and insulin resistance in familial Mediterranean fever. Eur J Rheumatol 2014;1(4):140-3. https://doi.org/10.5152/eurjrheumatol.2014.140045.
Onur H, Aral H, Arica V, Bercem GA, Kasapcopur O. Vitamin D levels in children with familial Mediterranean fever. Pediatr Rheumatol Online J 2016;14(1):28. https://doi.org/10.1186/s12969-016-0089-1.
Erten S, Altunoglu A, Ceylan GG, Maras Y, Koca C, Yüksel A. Low plasma Vitamin D levels in patients with familial Mediterranean fever. Rheumatol Int 2012;32(12):3845-9. https://doi.org/10.1007/s00296-011-2281-4.
Anik A, Catli G, Makay B, Abaci A, Küme T, Unsal E, et al. Decreased Vitamin D levels in children with familial Mediterranean fever. Int J Rheum Dis 2014;17(3):321-6. https://doi.org/10.1111/1756-185X.12253.
Kisacik B, Kaya SU, Pehlivan Y, Tasliyurt T, Sayarlioglu M, Onat AM. Decreased Vitamin D levels in patients with familial mediterranean fever. Rheumatol Int 2013;33(5):1355-7. https://doi.org/10.1007/s00296-011-2278-z.
Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum 1997;40(10):1879-85. https://doi.org/10.1002/art.1780401023.
Hollis BW, Wagner CL. Normal serum Vitamin D levels. N Engl J Med 2005;352(5):515-6. https://doi.org/10.1056/NEJM200502033520521.
Fradkin A, Yahav J, Zemer D, Jonas A. Colchicine-induced lactose malabsorption in patients with familial Mediterranean fever. Isr J Med Sci 1995;31(10):616-20.
Webb DI, Chodos RB, Mahar CQ, Faloon WW. Mechanism of vitamin B12 malabsorption in patients receiving colchicine. N Engl J Med 1968;279(16):845-50. https://doi.org/10.1056/NEJM196810172791602.
Karatay S, Yildirim K, Karakuzu A, Kiziltunc A, Engin RI, Eren YB, et al. Vitamin D status in patients with Behcet’s disease. Clinics (Sao Paulo) 2011;66(5):721-3.
Song GG, Bae SC, Lee YH. Vitamin D receptor FokI, BsmI, and TaqI polymorphisms and susceptibility to rheumatoid arthritis: A meta-analysis. Z Rheumatol 2016;75(3):322-9. https://doi.org/10.1007/s00393-015-1581-6.
Ateş Ö, Dölek B, Dalyan Y, Sarıkaya AT. Vitamin D receptor gene polymorphisms in rheumatoid arthritis. Arch Rheumatol 2011;26(2):145-9. https://doi.org/10.5152/tjr.2011.021.
Carvalho C, Marinho A, Leal B, Bettencourt A, Boleixa D, Almeida I, et al. Association between Vitamin D receptor (VDR) gene polymorphisms and systemic lupus erythematosus in Portuguese patients. Lupus 2015;24(8):846-53. https://doi.org/10.1177/0961203314566636.
Kolahi S, Khabbazi A, Khodadadi H, Estiar MA, Hajialiloo M, Emrahi L, et al. Vitamin D receptor gene polymorphisms in Iranian Azary patients with Behçet’s disease. Scand J Rheumatol 2015;44(2):163-7. https://doi.org/10.3109/03009742.2014.945477.
Kizildag S, Dedemoglu F, Anik A, Catli G, Kizildag S, Abaci A, et al. Association between Vitamin D receptor polymorphism and familial mediterranean fever disease in Turkish children. Biochem Genet 2016;54(2):169-76. https://doi.org/10.1007/s10528-015-9710-0.
Bodoki L, Chen JQ, Zeher M, Nagy-Vincze M, Griger Z, Zilahi E, et al. Vitamin D receptor gene polymorphisms and haplotypes in Hungarian patients with idiopathic inflammatory myopathy. Biomed Res Int 2015;2015:809895. https://doi.org/10.1155/2015/809895.
Ban Y, Taniyama M, Ban Y. Vitamin D receptor gene polymorphism is associated with Graves’ disease in the Japanese population. J Clin Endocrinol Metab 2000;85(12):4639-43. https://doi.org/10.1210/jcem.85.12.7038.
Hajj A, Chedid R, Chouery E, Megarbané A, Helene M, Yared G, et al. Relationship between Vitamin D receptor gene polymorphisms, cardiovascular risk factors and adiponectin in a healthy young population. Pharmacogenomics 2016;17(15):1675-86. https://doi.org/10.2217/pgs-2016-0045.
Kasifoglu T, Bilge SY, Sari I, Solmaz D, Senel S, Emmungil H, et al. Amyloidosis and its related factors in Turkish patients with familial Mediterranean fever: A multicenter study. Rheumatol (Oxford) 2014;53(4):741-5. ttps://doi.org/10.1093/rheumatology/ket400.
Saatçi U, Ozen S, Ozdemir S, Bakkaloglu A, Besbas N, Topaloglu R, et al. Familial Mediterranean fever in children: Report of a large series and discussion of the risk and prognostic factors of amyloidosis. Eur J Pediatr 1997;156(8):619-23. https://doi.org/10.1007/s004310050677.
Rashid MU, Muzaffar M, Khan FA, Kabisch M, Muhammad N, Faiz S, et al. Association between the BsmI Polymorphism in the Vitamin D receptor gene and breast cancer risk: Results from a Pakistani case-control study. PLoS One 2015;10(10):e0141562. https://doi.org/10.1371/journal.pone.0141562.
Azab SF, Ali YF, Farghaly MA, Hamed ME, Allah MA, Emam AA, et al. Vitamin D receptor gene BsmI polymorphisms in Egyptian children and adolescents with systemic lupus erythematosus: A case-control study. Medicine (Baltimore) 2016;95(46):e5233. https://doi.org/10.1097/MD.0000000000005233.
Kaleta B, Bogaczewicz J, Robak E, Sysa-Jedrzejowska A, Wrzoesk M, Szubierajska W, et al. Vitamin D receptor gene BsmI polymorphism in Polish patients with Systemic lupus erythematosus. ISRN Endocriol 2013;2013:427818. https://doi.org/ 10. 1155/2013/427818.
Akdogan A, Calguneri M, Yavuz B, Arslan EB, Kalyoncu U, Sahiner L, et al. Are familial Mediterranean fever (FMF) patients at increased risk for atherosclerosis? Impaired endothelial function and increased intima media thickness are found in FMF. J Am Coll Cardiol 2006;48(11):2351-3. https://doi.org/10.1016/j.jacc.2006.09.013.
Tanne D, Yaari S, Goldbourt U. High-density lipoprotein cholesterol and risk of ischemic stroke mortality. A 21-year follow-up of 8586 men from the Israeli Ischemic Heart Disease Study. Stroke 1997;28(1):83-7. https://doi.org/10.1161/01.STR.28.1.83.
Assmann G, Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience). Prospective Cardiovascular Münster study. Am J Cardiol 1992;70(7):733-7.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-08-06
Published 2018-02-20









