Non-endoscopic minimally invasive evacuation of intracerebral hematoma (ICH): A case report
DOI:
https://doi.org/10.17305/bjbms.2017.2289Keywords:
Intracerebral hemorrhage, ICH, bleeding disorders, hematoma evacuation, minimally invasive surgeryAbstract
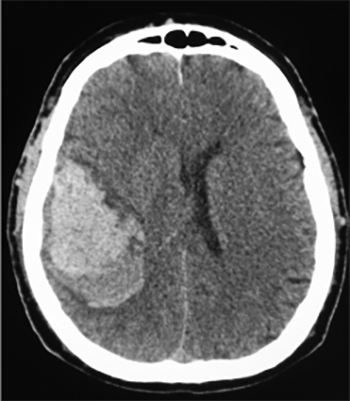
Spontaneous intracerebral hemorrhage (ICH) is one of the most serious causes of stroke, leading to high rates of disability and mortality. In addition to intensive medical treatment, surgery may help to improve the prognosis in patients with ICH. A rapid reversal of coagulopathy is essential in these patients, although it may be difficult to achieve in various bleeding disorders. In such cases, when surgery is needed, a minimally invasive approach is recommended. In this case report, we described and shortly discussed the evacuation of ICH with a minimally invasive non-endoscopic surgical technique.
Citations
Downloads
References
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol 2010;9(2):167-76. https://doi.org/10.1016/S1474-4422(09)70340-0.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009;373(9675):1632-44. https://doi.org/10.1016/S0140-6736(09)60371-8.
Godoy DA, Piñero GR, Koller P, Masotti L, Di Napoli M. Steps to consider in the approach and management of critically ill patient with spontaneous intracerebral hemorrhage. World J Crit Care Med 2015;4(3):213-29. DOI: 10.5492/wjccm.v4.i3.213.
Flaherty ML, Haverbusch M, Sekar P, Kissela B, Kleindorfer D, Moomaw CJ, et al. Long-term mortality after intracerebral hemorrhage. Neurology 2006;66(8):1182-6. https://doi.org/10.1212/01.wnl.0000208400.08722.7c.
Zheng J, Li H, Guo R, Lin S, Hu X, Dong W, et al. Minimally invasive surgery treatment for the patients with spontaneous supratentorial intracerebral hemorrhage (MISTICH): Protocol of a multi-center randomized controlled trial. BMC Neurol 2014;14:206. https://doi.org/10.1186/s12883-014-0206-z.
Santalucia P. Intracerebral hemorrhage: Medical treatment. Neurol Sci 2008;29 Suppl 2:S271-3. DOI: 10.1007/s10072-008-0961-y.
Chan S, Hemphill JC 3rd. Critical care management of intracerebral hemorrhage. Crit Care Clin 2014;30(4):699-717. https://doi.org/10.1016/j.ccc.2014.06.003.
Delcourt C, Huang Y, Arima H, Chalmers J, Davis SM, Heeley EL, et al. Hematoma growth and outcomes in intracerebral hemorrhage: The INTERACT1 study. Neurology 2012;79(4):314-9. https://doi.org/10.1212/WNL.0b013e318260cbba.
Miller CM, Vespa P, Saver JL, Kidwell CS, Carmichael ST, Alger J, et al. Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol 2008;69(5):441-6. https://doi.org/10.1016/j.surneu.2007.12.016.
Arboix A, Comes E, García-Eroles L, Massons J, Oliveres M, Balcells M, et al. Site of bleeding and early outcome in primary intracerebral hemorrhage. Acta Neurol Scand 2002;105(4):282-8. https://doi.org/10.1034/j.1600-0404.2002.1o170.x.
Ritter MA, Droste DW, Hegedüs K, Szepesi R, Nabavi DG, Csiba L, et al. Role of cerebral amyloid angiopathy in intracerebral hemorrhage in hypertensive patients. Neurology 2005;64(7):1233-7. https://doi.org/10.1212/01.WNL.0000156522.93403.C3.
Arboix A, Vall-Llosera A, García-Eroles L, Massons J, Oliveres M, Targa C. Clinical features and functional outcome of intracerebral hemorrhage in patients aged 85 and older. J Am Geriatr Soc 2002;50(3):449-54. https://doi.org/10.1046/j.1532-5415.2002.50109.x.
Andrews CM, Jauch EC, Hemphill JC 3rd, Smith WS, Weingart SD. Emergency neurological life support: Intracerebral hemorrhage. Neurocrit Care 2012;17 Suppl 1:S37-46. DOI: 10.1007/s12028-012-9757-2.
Steiner T, Bösel J. Options to restrict hematoma expansion after spontaneous intracerebral hemorrhage. Stroke 2010;41(2):402-9. https://doi.org/10.1161/STROKEAHA.109.552919.
Chen CC, Lin HL, Cho DY. Endoscopic surgery for thalamic hemorrhage: A technical note. Surg Neurol 2007;68(4):438-42. https://doi.org/10.1016/j.surneu.2006.11.054.
Rennert RC, Signorelli JW, Abraham P, Pannell JS, Khalessi AA. Minimally invasive treatment of intracerebral hemorrhage. Expert Rev Neurother 2015;15(8):919-33. DOI: 10.1586/14737175.2015.1059755.
Duff TA, Ayeni S, Levin AB, Javid M. Nonsurgical management of spontaneous intracerebral hematoma. Neurosurgery 1981;9(4):387-93. https://doi.org/10.1227/00006123-198110000-00007.
Talukder MM, Islam KM, Hossain M, Jahan MU, Mahmood E, Hossain SS. Surgery for primary intracerebral haemorrhage: Is it safe and effective? Bangladesh Med Res Counc Bull 2012;38(3):74-8.
Agmazov MK, Bersnev VP, Ivanova NE, Pavlov OA, Nikitin AI, Akhtamov DA, et al. Minimally invasive surgery of patients with hypertensive intracerebral bleedings. [Article in Russian]. Vestn Khir Im I I Grek 2009;168(2):78-82.
Yamashiro S, Hitoshi Y, Yoshida A, Kuratsu J. Effectiveness of endoscopic surgery for comatose patients with large supratentorial intracerebral hemorrhages. Neurol Med Chir (Tokyo) 2015;55(11):819-23. https://doi.org/10.2176/nmc.oa.2014-0136.
Wang WH, Hung YC, Hsu SP, Lin CF, Chen HH, Shih YH, et al. Endoscopic hematoma evacuation in patients with spontaneous supratentorial intracerebral hemorrhage. J Chin Med Assoc 2015;78(2):101-7. https://doi.org/10.1016/j.jcma.2014.08.013.
Anegawa S, Torigoe R, Hayashi T, Yamashita Y, Furukawa Y. A case of primary fibrosarcoma caused by spontaneous intracerebral hematoma. No Shinkei Geka 1991;19(5):485-90.
Carvi y Nievas MN. Why, when, and how spontaneous intracerebral hematomas should be operated. Med Sci Monit 2005;11(1):RA24-31.
Fernandes HM, Gregson B, Siddique S, Mendelow AD. Surgery in intracerebral hemorrhage. The uncertainty continues. Stroke 2000;31(10):2511-6. https://doi.org/10.1161/01.STR.31.10.2511.
Additional Files
Published
How to Cite
Accepted 2017-08-06
Published 2018-11-07









