Calcitonin is expressed in the submaxillary glands of rats
DOI:
https://doi.org/10.17305/bjbms.2014.2294Keywords:
submaxillary gland, calcitonin, immunocytochemistry, nucleic acid hybridizationAbstract
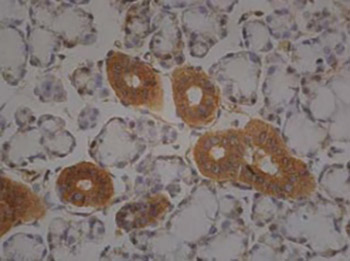
Calcitonin is usually produced by the parafollicular cells of the thyroid. However in an immunohistochemistry experiment we observed that the cells of the serous acini of rat submaxillary gland tissue were stained positive with calcitonin antibodies. We further used immunocytochemistry and nucleic acid hybridization to localize the distribution of calcitonin protein and calcitonin mRNA respectively in cultivated cells of rat submaxillary glands. The results showed that the cytoplasm of the epithelial cells of the submaxillary glands had positive staining in immunocytochemistry using calcitonin monoclonal antibody and positive reaction in nucleic acid hybridization using calcitonin mRNA complementary DNA probe. For the first time we found that the cells of the submaxillary glands of rats can produce the hormone calcitonin.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-07-19
Published 2014-05-20









